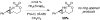A chiral rhodium carboxamidate catalyst for enantioselective C-H amination
- PMID: 18582043
- PMCID: PMC2597189
- DOI: 10.1021/ja8031955
A chiral rhodium carboxamidate catalyst for enantioselective C-H amination
Abstract
Rh2(S-nap)4, a chiral dirhodium tetracarboxamidate complex, has been developed and shown to be an effective catalyst for the asymmetric, intramolecular C-H amination of sulfamate esters. Enantiomeric excesses range from 60-99% for a collection of disparately substituted 3-arylpropylsulfamates. In addition, Rh2(S-nap)4 is found to promote chemoselective allylic C-H oxidation of unsaturated sulfamates, a property not observed with other dirhodium complexes tested to date.
Figures
References
-
-
For a general reference to reactions of diazoalkanes, see: Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides. Wiley and Sons, Ltd.; New York: 1997. .
-
-
- Yamawaki M, Kitagaki S, Anada M, Hashimoto S. Heterocycles. 2006;69:527.
- Zhang J, Chan PWH, Che C-M. Tetrahedron Lett. 2005;46:5403.
- Fruit C, Müller P. Helv. Chim. Acta. 2004;87:1607.
- Liang J-L, Yuan S-X, Chan PWH, Che C-M. Tetrahedron Lett. 2003;44:5917.
- Liang JL, Yuan S-X, Huang J-S, Yu W-Y, Che C-M. Angew. Chem., Int. Ed. 2002;41:3465. - PubMed
- Yamawaki M, Tsutsui H, Kitagaki S, Anada M, Hashimoto S. Tetrahedron Lett. 2002;43:9561.
-
- Reddy RP, Davies HML. Org. Lett. 2006;8:5016.
- Omura K, Murakami M, Uchida T, Irie R, Katsuki T. Chem. Lett. 2003;32:354.
- Kohmura Y, Katsuki T. Tetrahedron Lett. 2001;42:3339.
-
-
For a diastereoselective intermolecular C—H amination catalyzed by a chiral dirhodium complex, see: Liang C, Collet F, Robert-Peillard F, Müller P, Dodd RH, Dauban P. J. Am. Chem. Soc. 2008;130:343.. Liang C, Robert-Peillard F, Fruit M, Müller P, Dodd RH, Dauban P. Angew. Chem., Int. Ed. 2006;45:4641..
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials