Synthesis and photophysical investigation of squaraine rotaxanes by "clicked capping"
- PMID: 18582079
- PMCID: PMC2848988
- DOI: 10.1021/ol801189a
Synthesis and photophysical investigation of squaraine rotaxanes by "clicked capping"
Abstract
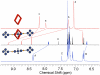
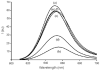
Pseudorotaxane complexes of squaraine dyes and tetralactam macrocycles are converted into permanently interlocked rotaxane structures using copper-catalyzed and copper-free cycloaddition reactions with bulky stopper groups. The photophysical properties of the encapsulated squaraine depend on the structure of the macrocycle. In one case, squaraine rotaxanes are produced in near-quantitative yields and with intense near-IR fluorescence. In another case, squaraine fluorescence is greatly diminished upon macrocyclic encapsulation but the signal can be restored by dye displacement with anions.
Figures

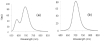


Similar articles
-
Internal and external stereoisomers of squaraine rotaxane endoperoxide: synthesis, chemical differences, and structural revision.J Org Chem. 2014 Feb 7;79(3):1120-30. doi: 10.1021/jo402564k. J Org Chem. 2014. PMID: 24428682
-
Squaraine rotaxanes with boat conformation macrocycles.J Org Chem. 2009 Sep 4;74(17):6462-8. doi: 10.1021/jo901298n. J Org Chem. 2009. PMID: 19639940 Free PMC article.
-
Discovery and early development of squaraine rotaxanes.Chem Commun (Camb). 2009 Nov 14;(42):6329-38. doi: 10.1039/b911064j. Epub 2009 Aug 24. Chem Commun (Camb). 2009. PMID: 19841772 Free PMC article.
-
Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges.Bioconjug Chem. 2020 Feb 19;31(2):194-213. doi: 10.1021/acs.bioconjchem.9b00482. Epub 2019 Aug 12. Bioconjug Chem. 2020. PMID: 31365819 Free PMC article. Review.
-
Anion-templated assembly of interpenetrated and interlocked structures.Chem Commun (Camb). 2006 May 28;(20):2105-17. doi: 10.1039/b516435b. Epub 2006 Mar 8. Chem Commun (Camb). 2006. PMID: 16703125 Review.
Cited by
-
New tetralactam hosts for squaraine dyes.Org Biomol Chem. 2018 Nov 28;16(46):8976-8983. doi: 10.1039/c8ob02596g. Org Biomol Chem. 2018. PMID: 30418456 Free PMC article.
-
Coupled molecular switching processes in ordered mono- and multilayers of stimulus-responsive rotaxanes on gold surfaces.J Am Chem Soc. 2015 Apr 8;137(13):4382-90. doi: 10.1021/ja512654d. Epub 2015 Mar 26. J Am Chem Soc. 2015. PMID: 25782057 Free PMC article.
-
Efficient synthesis of fluorescent squaraine rotaxane dendrimers.Org Lett. 2010 Jan 1;12(1):140-3. doi: 10.1021/ol902546m. Org Lett. 2010. PMID: 19957971 Free PMC article.
-
Microwave-assisted slipping synthesis of fluorescent squaraine rotaxane probe for bacterial imaging.Chem Commun (Camb). 2010 Feb 21;46(7):1068-9. doi: 10.1039/b924350j. Epub 2010 Jan 13. Chem Commun (Camb). 2010. PMID: 20126715 Free PMC article.
-
Effect of backbone flexibility on covalent template-directed synthesis of linear oligomers.Org Biomol Chem. 2022 Nov 2;20(42):8285-8292. doi: 10.1039/d2ob01627c. Org Biomol Chem. 2022. PMID: 36226964 Free PMC article.
References
-
- Shen X, Van Wijk R, editors. Biophotonics: Optical Science and Engineering for the 21st Century. Springer; New York: 2005.
- Cox GC, editor. Optical Imaging Techniques in Cell Biology. CRC Press; Boca Raton: 2007.
- Wolfbeis OS. Fluorescence Methods and Applications: Spectroscopy, Imaging, and Probes. Wiley-Blackwell; New York: 2008.
-
- Arunkumar E, Forbes CC, Smith BD. Eur. J. Org. Chem. 2005:4051–4059.
- Buston JEH, Young JR, Anderson HL. Chem. Commun. 2000:905–906.
- Park JS, Wilson JN, Hardcastle KI, Bunz UHF, Srinivasarao M. J. Am. Chem. Soc. 2006;128:7714–7715. - PubMed
- Mohanty J, Pal H, Ray AK, Kumar S, Nau WM. ChemPhysChem. 2007;8:54–56. - PubMed
- Comes M, Marcos MD, Martínez-Máñez R, Millán MC, Ros-Lis JV, Sancenón F, Soto J, Villaescusa LA. Chem. Eur. J. 2006;12:2162–2170. - PubMed
- Constantin TP, Silva GL, Robertson KL, Hamilton TP, Fague K, Waggoner AS, Armitage BA. Org. Lett. 2008;10:1561–1564. - PubMed
-
- Arunkumar E, Forbes CC, Noll BC, Smith BD. J. Am. Chem. Soc. 2005;127:3288–3289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

