Neuroendocrine inhibition of glucose production and resistance to cancer in dwarf mice
- PMID: 18582556
- PMCID: PMC2872123
- DOI: 10.1016/j.exger.2008.05.014
Neuroendocrine inhibition of glucose production and resistance to cancer in dwarf mice
Abstract
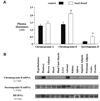
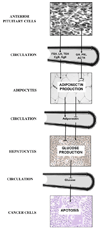
Pit1 null (Snell dwarf) and Proph1 null (Ames dwarf) mutant mice lack GH, PRL and TSH. Snell and Ames dwarf mice also exhibit reduced IGF-I, resistance to cancer and a longer lifespan than control mice. Endogenous glucose production during fasting is reduced in Snell dwarf mice compared to fasting control mice. In view of cancer cell dependence on glucose for energy, low endogenous glucose production may provide Snell dwarf mice with resistance to cancer. We investigated whether endogenous glucose production is lower in Snell dwarf mice during feeding. Inhibition of endogenous glucose production by glucose injection was enhanced in 12 to 14 month-old female Snell dwarf mice. Thus, we hypothesize that lower endogenous glucose production during feeding and fasting reduces cancer cell glucose utilization providing Snell dwarf mice with resistance to cancer. The elevation of circulating adiponectin, a hormone produced by adipose tissue, may contribute to the suppression of endogenous glucose production in 12 to 14 month-old Snell dwarf mice. We compared the incidence of cancer at time of death between old Snell dwarf and control mice. Only 18% of old Snell dwarf mice had malignant lesions at the time of death compared to 82% of control mice. The median ages at death for old Snell dwarf and control mice were 33 and 26 months, respectively. By contrast, previous studies showed a high incidence of cancer in old Ames dwarf mice at the time of death. Hence, resistance to cancer in old Snell dwarf mice may be mediated by neuroendocrine factors that reduce glucose utilization besides elevated adiponectin, reduced IGF-I and a lack of GH, PRL and TSH, seen in both Snell and Ames dwarf mice. Proteomics analysis of pituitary secretions from Snell dwarf mice confirmed the absence of GH and PRL, the secretion of ACTH and elevated secretion of Chromogranin B and Secretogranin II. Radioimmune assays confirmed that circulating Chromogranin B and Secretogranin II were elevated in 12 to 14 month-old Snell dwarf mice. In summary, our results in Snell dwarf mice suggest that the pituitary gland and adipose tissue are part of a neuroendocrine loop that lowers the risk of cancer during aging by reducing the availability of glucose.
Figures



Similar articles
-
Reduced hypothalamic neuropeptide Y expression in growth hormone- and prolactin-deficient Ames and Snell dwarf mice.Endocrinology. 2003 Nov;144(11):4783-9. doi: 10.1210/en.2003-0753. Epub 2003 Aug 14. Endocrinology. 2003. PMID: 12960004
-
Body composition of prolactin-, growth hormone, and thyrotropin-deficient Ames dwarf mice.Endocrine. 2003 Feb-Mar;20(1-2):149-54. doi: 10.1385/ENDO:20:1-2:149. Endocrine. 2003. PMID: 12668880
-
Postnatal regression of hypothalamic dopaminergic neurons in prolactin-deficient Snell dwarf mice.Endocrinology. 2004 Dec;145(12):5656-64. doi: 10.1210/en.2004-0931. Epub 2004 Sep 2. Endocrinology. 2004. PMID: 15345680
-
Life extension in the dwarf mouse.Curr Top Dev Biol. 2004;63:189-225. doi: 10.1016/S0070-2153(04)63006-7. Curr Top Dev Biol. 2004. PMID: 15536017 Review.
-
Pituitary hormones as neurotrophic signals: update on hypothalamic differentiation in genetic models of altered feedback.Proc Soc Exp Biol Med. 1999 Oct;222(1):39-58. doi: 10.1111/j.1525-1373.1999.09994.x. Proc Soc Exp Biol Med. 1999. PMID: 10510245 Review.
Cited by
-
Growth hormone and aging.Rev Endocr Metab Disord. 2021 Mar;22(1):71-80. doi: 10.1007/s11154-020-09593-2. Epub 2020 Oct 1. Rev Endocr Metab Disord. 2021. PMID: 33001358 Review.
-
Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity?J Endocrinol. 2013 Feb 25;216(3):363-74. doi: 10.1530/JOE-12-0505. Print 2013 Mar. J Endocrinol. 2013. PMID: 23261955 Free PMC article.
-
Transcriptome analysis in calorie-restricted rats implicates epigenetic and post-translational mechanisms in neuroprotection and aging.Genome Biol. 2015 Dec 22;16:285. doi: 10.1186/s13059-015-0847-2. Genome Biol. 2015. PMID: 26694192 Free PMC article.
-
Health Benefits of Fasting and Caloric Restriction.Curr Diab Rep. 2017 Oct 23;17(12):123. doi: 10.1007/s11892-017-0951-7. Curr Diab Rep. 2017. PMID: 29063418 Review.
-
Mouse models of growth hormone deficiency.Rev Endocr Metab Disord. 2021 Mar;22(1):3-16. doi: 10.1007/s11154-020-09601-5. Epub 2020 Oct 9. Rev Endocr Metab Disord. 2021. PMID: 33033978 Review.
References
-
- Argentino DP, Dominici FP, Munoz MC, Al-Regaiey K, Bartke A, Turyn D. Effects of long-term caloric restriction on glucose homeostasis and on the first steps of the insulin signaling system in skeletal muscle of normal and Ames dwarf (Prop1df/Prop1df) mice. Exp. Gerontol. 2005;40:27–35. - PubMed
-
- Asa SL, Puy LA, Lew AM, Sundmark VC, Elsholtz HP. Cell type-specific expression of the pituitary transcription activator pit-1 in the human pituitary and pituitary adenomas. J. Clin. Endocrinol. Metab. 1993;77:1275–1280. - PubMed
-
- Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes. 2005;54:1942–1948. - PubMed
-
- Beard JC, Bergman RN, Ward WK, Porte D., Jr The insulin sensitivity index in nondiabetic man. Correlation between clamp-derived and IVGTT-derived values. Diabetes. 1986;35:362–369. - PubMed
-
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

