Characterization and use of laser-based lysis for cell analysis on-chip
- PMID: 18583277
- PMCID: PMC2706033
- DOI: 10.1098/rsif.2008.0177.focus
Characterization and use of laser-based lysis for cell analysis on-chip
Abstract
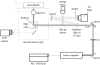
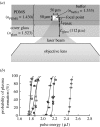
We demonstrate the use of a pulsed laser microbeam for cell lysis followed by electrophoretic separation of cellular analytes in a microfluidic device. The influence of pulse energy and laser focal point within the microchannel on the threshold for plasma formation was measured. The thickness of the poly(dimethylsiloxane) (PDMS) layer through which the beam travelled was a critical determinant of the threshold energy. An effective optical path length, Leff, for the laser beam can be used to predict the threshold for optical breakdown at different microchannel locations. A key benefit of laser-based cell lysis is the very limited zone (less than 5 microm) of lysis. A second asset is the rapid cell lysis times (approx. microseconds). These features enable two analytes, fluorescein and Oregon Green, from a cell to be electrophoretically separated in the channel in which cell lysis occurred. The resolution and efficiency of the separation of the cellular analytes are similar to those of standards demonstrating the feasibility of using a pulsed laser microbeam in single-cell analysis.
Figures



References
-
- Berridge M.J. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi:10.1038/361315a0 - DOI - PubMed
-
- Braschler T., Johann R., Heule M., Metref L., Renaud P. Gentle cell trapping and release on a microfluidic chip by in situ alginate hydrogel formation. Lab Chip. 2005;5:553–559. doi:10.1039/b417604a - DOI - PubMed
-
- Di Carlo D., Lee L.P. Dynamic single-cell analysis for quantitative biology. Anal. Chem. 2006;78:7918–7925. - PubMed
-
- Dovichi N.J., Hu S. Chemical cytometry. Curr. Opin. Chem. Biol. 2003;7:603–608. doi:10.1016/j.cbpa.2003.08.012 - DOI - PubMed
-
- Evander M., Johansson L., Lilliehorn T., Piskur J., Lindvall M., Johansson S., Almqvist M., Laurell T., Nilsson J. Noninvasive acoustic cell trapping in a microfluidic perfusion system for online bioassays. Anal. Chem. 2007;79:2984–2991. doi:10.1021/ac061576v - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
