Interplay between liquid crystalline and isotropic gels in self-assembled neurofilament networks
- PMID: 18583309
- PMCID: PMC2440473
- DOI: 10.1529/biophysj.107.127415
Interplay between liquid crystalline and isotropic gels in self-assembled neurofilament networks
Abstract
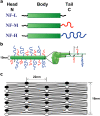
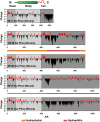
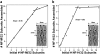
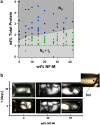
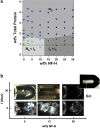
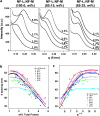
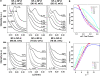
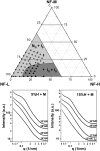
Neurofilaments (NFs) are a major constituent of nerve cell axons that assemble from three subunit proteins of low (NF-L), medium (NF-M), and high (NF-H) molecular weight into a 10 nm diameter rod with radiating sidearms to form a bottle-brush-like structure. Here, we reassemble NFs in vitro from varying weight ratios of the subunit proteins, purified from bovine spinal cord, to form homopolymers of NF-L or filaments composed of NF-L and NF-M (NF-LM), NF-L and NF-H (NF-LH), or all three subunits (NF-LMH). At high protein concentrations, NFs align to form a nematic liquid crystalline gel with a well-defined spacing determined with synchrotron small angle x-ray scattering. Near physiological conditions (86 mM monovalent salt and pH 6.8), NF-LM networks with a high NF-M grafting density favor nematic ordering whereas filaments composed of NF-LH transition to an isotropic gel at low protein concentrations as a function of increasing mole fraction of NF-H subunits. The interfilament distance decreases with NF-M grafting density, opposite the trend seen with NF-LH networks. This suggests a competition between the more attractive NF-M sidearms, forming a compact aligned nematic gel, and the repulsive NF-H sidearms, favoring a more expansive isotropic gel, at 86 mM monovalent salt. These interactions are highly salt dependent and the nematic gel phase is stabilized with increasing monovalent salt.
Figures

Similar articles
-
Gel-expanded to gel-condensed transition in neurofilament networks revealed by direct force measurements.Nat Mater. 2010 Jan;9(1):40-6. doi: 10.1038/nmat2566. Epub 2009 Nov 15. Nat Mater. 2010. PMID: 19915555
-
Neurofilament networks: Salt-responsive hydrogels with sidearm-dependent phase behavior.Biochim Biophys Acta. 2016 Jul;1860(7):1560-9. doi: 10.1016/j.bbagen.2016.03.018. Epub 2016 Mar 16. Biochim Biophys Acta. 2016. PMID: 26993199
-
Neurofilament sidearms modulate parallel and crossed-filament orientations inducing nematic to isotropic and re-entrant birefringent hydrogels.Nat Commun. 2013;4:2224. doi: 10.1038/ncomms3224. Nat Commun. 2013. PMID: 23892390
-
Structures and interactions in 'bottlebrush' neurofilaments: the role of charged disordered proteins in forming hydrogel networks.Biochem Soc Trans. 2012 Oct;40(5):1027-31. doi: 10.1042/BST20120101. Biochem Soc Trans. 2012. PMID: 22988859 Review.
-
Liquid-crystalline physical gels.Chem Soc Rev. 2007 Dec;36(12):1857-67. doi: 10.1039/b612546h. Epub 2007 Sep 3. Chem Soc Rev. 2007. PMID: 17982513 Review.
Cited by
-
Minireview - Microtubules and Tubulin Oligomers: Shape Transitions and Assembly by Intrinsically Disordered Protein Tau and Cationic Biomolecules.Langmuir. 2019 Dec 3;35(48):15970-15978. doi: 10.1021/acs.langmuir.9b02208. Epub 2019 Oct 2. Langmuir. 2019. PMID: 31539262 Free PMC article. Review.
-
Phosphorylation-Induced Mechanical Regulation of Intrinsically Disordered Neurofilament Proteins.Biophys J. 2017 Mar 14;112(5):892-900. doi: 10.1016/j.bpj.2016.12.050. Biophys J. 2017. PMID: 28297648 Free PMC article.
-
Liquid crystal assemblies in biologically inspired systems.Liq Cryst. 2013 Jan 1;40(12):1748-1758. doi: 10.1080/02678292.2013.846422. Liq Cryst. 2013. PMID: 24558293 Free PMC article.
-
The specificity of the interaction between αB-crystallin and desmin filaments and its impact on filament aggregation and cell viability.Philos Trans R Soc Lond B Biol Sci. 2013 Mar 25;368(1617):20120375. doi: 10.1098/rstb.2012.0375. Print 2013 May 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23530264 Free PMC article.
-
Nanoscale assembly in biological systems: from neuronal cytoskeletal proteins to curvature stabilizing lipids.Adv Mater. 2011 May 24;23(20):2260-70. doi: 10.1002/adma.201004647. Epub 2011 Apr 20. Adv Mater. 2011. PMID: 21506171 Free PMC article. Review.
References
-
- Cohlberg, J. A., H. Hajarian, T. Tran, P. Alipourjeddi, and A. Noveen. 1995. Neurofilament protein heterotetramers as assembly intermediates. J. Biol. Chem. 270:9334–9339. - PubMed
-
- Geisler, N., and K. Weber. 1981. Self-assembly in vitro of the 68,000 molecular weight component of the mammalian neurofilament triplet proteins into intermediate-sized filaments. J. Mol. Biol. 151:565–571. - PubMed
-
- Fuchs, E., and D. W. Cleveland. 1998. A structural scaffolding of intermediate filaments in health and disease. Science. 279:514–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

