Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction
- PMID: 18583310
- PMCID: PMC2475782
- DOI: 10.2353/ajpath.2008.071193
Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction
Abstract
Unexplained intrauterine growth restriction of the fetus (IUGR) results from impaired placental development, frequently associated with maternal malperfusion. Some cases are complicated further by preeclampsia (PE+IUGR). Here, we provide the first evidence that placental protein synthesis inhibition and endoplasmic reticulum (ER) stress play key roles in IUGR pathophysiology. Increased phosphorylation of eukaryotic initiation factor 2alpha suggests suppression of translation initiation in IUGR placentas, with a further increase in PE+IUGR cases. Consequently, AKT levels were reduced at the protein, but not mRNA, level. Additionally, levels of other proteins in the AKT-mammalian target of rapamycin pathway were decreased, and there was associated dephosphorylation of 4E-binding protein 1 and activation of glycogen synthase kinase 3beta. Cyclin D1 and the eukaryotic initiation factor 2B epsilon subunit were also down-regulated, providing additional evidence for this placental phenotype. The central role of AKT signaling in placental growth regulation was confirmed in Akt1 null mice, which display IUGR. In addition, we demonstrated ultrastructural and molecular evidence of ER stress in human IUGR and PE+IUGR placentas, providing a potential mechanism for eukaryotic initiation factor 2alpha phosphorylation. In confirmation, induction of low-grade ER stress in trophoblast-like cell lines reduced cellular proliferation. PE+IUGR placentas showed elevated ER stress with the additional expression of the pro-apoptotic protein C/EBP-homologous protein/growth arrest and DNA damage 153. These findings may account for the increased microparticulate placental debris in the maternal circulation of these cases, leading to endothelial cell activation and impairing placental development.
Figures
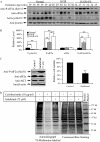
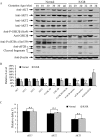

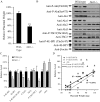

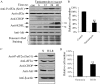
Comment in
-
The endoplasmic reticulum stress of placental impoverishment.Am J Pathol. 2008 Aug;173(2):311-4. doi: 10.2353/ajpath.2008.080412. Am J Pathol. 2008. PMID: 18653777 Free PMC article.
Similar articles
-
Endoplasmic reticulum stress disrupts placental morphogenesis: implications for human intrauterine growth restriction.J Pathol. 2012 Dec;228(4):554-64. doi: 10.1002/path.4068. Epub 2012 Sep 28. J Pathol. 2012. PMID: 22733590 Free PMC article.
-
Intrauterine growth restriction in humans is associated with abnormalities in placental insulin-like growth factor signaling.Endocrinology. 2005 Mar;146(3):1498-505. doi: 10.1210/en.2004-1332. Epub 2004 Nov 24. Endocrinology. 2005. PMID: 15564321
-
No evidence of the unfolded protein response in the placenta of two rodent models of preeclampsia and intrauterine growth restriction.Biol Reprod. 2021 Aug 3;105(2):449-463. doi: 10.1093/biolre/ioab087. Biol Reprod. 2021. PMID: 33955453
-
Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia.Placenta. 2009 Mar;30 Suppl A(Suppl):S43-8. doi: 10.1016/j.placenta.2008.11.003. Epub 2008 Dec 9. Placenta. 2009. PMID: 19081132 Free PMC article. Review.
-
Altered levels of insulin-like growth factor binding protein proteases in preeclampsia and intrauterine growth restriction.Prenat Diagn. 2010 Sep;30(9):815-20. doi: 10.1002/pd.2583. Prenat Diagn. 2010. PMID: 20658698 Review.
Cited by
-
Placental Function and the Development of Fetal Overgrowth and Fetal Growth Restriction.Obstet Gynecol Clin North Am. 2021 Jun;48(2):247-266. doi: 10.1016/j.ogc.2021.02.001. Obstet Gynecol Clin North Am. 2021. PMID: 33972064 Free PMC article. Review.
-
Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia.Pregnancy Hypertens. 2011 Jan;1(1-2):72-8. doi: 10.1016/j.preghy.2010.12.002. Pregnancy Hypertens. 2011. PMID: 22242213 Free PMC article.
-
Protein Misfolding in Pregnancy: Current Insights, Potential Mechanisms, and Implications for the Pathogenesis of Preeclampsia.Molecules. 2024 Jan 26;29(3):610. doi: 10.3390/molecules29030610. Molecules. 2024. PMID: 38338354 Free PMC article. Review.
-
Extracellularly Released Calreticulin Induced by Endoplasmic Reticulum Stress Impairs Syncytialization of Cytotrophoblast Model BeWo Cells.Cells. 2021 May 24;10(6):1305. doi: 10.3390/cells10061305. Cells. 2021. PMID: 34073978 Free PMC article.
-
Increased autophagy in placentas of intrauterine growth-restricted pregnancies.PLoS One. 2012;7(7):e40957. doi: 10.1371/journal.pone.0040957. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22815878 Free PMC article.
References
-
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182:198–206. - PubMed
-
- Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004;58:894–900. - PubMed
-
- Hafner E, Metzenbauer M, Hofinger D, Munkel M, Gassner R, Schuchter K, Dillinger-Paller B, Philipp K. Placental growth from the first to the second trimester of pregnancy in SGA-foetuses and pre-eclamptic pregnancies compared to normal foetuses. Placenta. 2003;24:336–342. - PubMed
-
- Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–663. - PubMed
-
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

