Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis
- PMID: 18583313
- PMCID: PMC2475778
- DOI: 10.2353/ajpath.2008.080142
Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis
Abstract
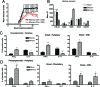
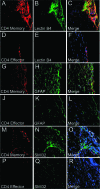
The persistence of human autoimmune diseases is thought to be mediated predominantly by memory T cells. We investigated the phenotype and migration of memory versus effector T cells in vivo in experimental autoimmune encephalomyelitis (EAE). We found that memory CD4(+) T cells up-regulated the activation marker CD44 as well as CXCR3 and ICOS, proliferated more and produced more interferon-gamma and less interleukin-17 compared to effector T cells. Moreover, adoptive transfer of memory T cells into T cell receptor (TCR)alphabeta(-/-) recipients induced more severe disease than did effector CD4(+) T cells with marked central nervous system inflammation and axonal damage. The uniqueness of disease mediated by memory T cells was confirmed by the differential susceptibility to immunomodulatory therapies in vivo. CD28-B7 T cell costimulatory signal blockade by CTLA4Ig suppressed effector cell-mediated EAE but had minimal effects on disease induced by memory cells. In contrast, ICOS-B7h blockade exacerbated effector T cell-induced EAE but protected from disease induced by memory T cells. However, blockade of the OX40 (CD134) costimulatory pathway ameliorated disease mediated by both memory and effector T cells. Our data extend the understanding of the pathogenicity of autoreactive memory T cells and have important implications for the development of novel therapies for human autoimmune diseases.
Figures


Similar articles
-
CD28-independent induction of experimental autoimmune encephalomyelitis.J Clin Invest. 2001 Mar;107(5):575-83. doi: 10.1172/JCI11220. J Clin Invest. 2001. PMID: 11238558 Free PMC article.
-
ICOS deficiency results in exacerbated IL-17 mediated experimental autoimmune encephalomyelitis.J Clin Immunol. 2009 Jul;29(4):426-33. doi: 10.1007/s10875-009-9287-7. Epub 2009 Mar 17. J Clin Immunol. 2009. PMID: 19291374
-
Age-associated changes in rat immune system: lessons learned from experimental autoimmune encephalomyelitis.Exp Gerontol. 2014 Oct;58:179-97. doi: 10.1016/j.exger.2014.08.005. Epub 2014 Aug 13. Exp Gerontol. 2014. PMID: 25128713
-
Targeting T cell costimulation in autoimmune disease.Expert Opin Ther Targets. 2002 Jun;6(3):275-89. doi: 10.1517/14728222.6.3.275. Expert Opin Ther Targets. 2002. PMID: 12223069 Review.
-
Differential requirements of naïve and memory T cells for CD28 costimulation in autoimmune pathogenesis.Histol Histopathol. 1999 Oct;14(4):1269-76. doi: 10.14670/HH-14.1269. Histol Histopathol. 1999. PMID: 10506942 Review.
Cited by
-
OX40L Inhibition Suppresses KLH-driven Immune Responses in Healthy Volunteers: A Randomized Controlled Trial Demonstrating Proof-of-Pharmacology for KY1005.Clin Pharmacol Ther. 2022 May;111(5):1121-1132. doi: 10.1002/cpt.2539. Epub 2022 Mar 1. Clin Pharmacol Ther. 2022. PMID: 35092305 Free PMC article. Clinical Trial.
-
Rheumatoid arthritis-associated RBPJ polymorphism alters memory CD4+ T cells.Hum Mol Genet. 2016 Jan 15;25(2):404-17. doi: 10.1093/hmg/ddv474. Epub 2015 Nov 24. Hum Mol Genet. 2016. PMID: 26604133 Free PMC article.
-
Enhancing the ability of experimental autoimmune encephalomyelitis to serve as a more rigorous model of multiple sclerosis through refinement of the experimental design.Comp Med. 2009 Apr;59(2):112-28. Comp Med. 2009. PMID: 19389303 Free PMC article.
-
Dynamic cross-regulation of antigen-specific effector and regulatory T cell subpopulations and microglia in brain autoimmunity.BMC Syst Biol. 2013 Apr 26;7:34. doi: 10.1186/1752-0509-7-34. BMC Syst Biol. 2013. PMID: 23618467 Free PMC article.
-
Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor: structure optimization and pharmacokinetics.J Pharmacol Exp Ther. 2010 Mar;332(3):1136-45. doi: 10.1124/jpet.109.161109. Epub 2009 Dec 21. J Pharmacol Exp Ther. 2010. PMID: 20026673 Free PMC article.
References
-
- Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. - PubMed
-
- Imitola J, Chitnis T, Khoury SJ. Insights into the molecular pathogenesis of progression in multiple sclerosis: potential implications for future therapies. Arch Neurol. 2006;63:25–33. - PubMed
-
- Weiner HL. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol. 2004;61:1613–1615. - PubMed
-
- Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–984. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

