Growth hormone promotes lymphangiogenesis
- PMID: 18583315
- PMCID: PMC2475794
- DOI: 10.2353/ajpath.2008.080060
Growth hormone promotes lymphangiogenesis
Abstract
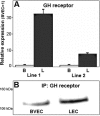
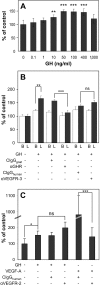
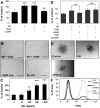
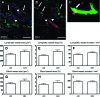
The lymphatic system plays an important role in inflammation and cancer progression, although the molecular mechanisms involved are poorly understood. As determined using comparative transcriptional profiling studies of cultured lymphatic endothelial cells versus blood vascular endothelial cells, growth hormone receptor was expressed at much higher levels in lymphatic endothelial cells than in blood vascular endothelial cells. These findings were confirmed by quantitative real-time reverse transcriptase-polymerase chain reaction and Western blot analyses. Growth hormone induced in vitro proliferation, sprouting, tube formation, and migration of lymphatic endothelial cells, and the mitogenic effect was independent of vascular endothelial growth factor receptor-2 or -3 activation. Growth hormone also inhibited serum starvation-induced lymphatic endothelial cell apoptosis. No major alterations of lymphatic vessels were detected in the normal skin of bovine growth hormone-transgenic mice. However, transgenic delivery of growth hormone accelerated lymphatic vessel ingrowth into the granulation tissue of full-thickness skin wounds, and intradermal delivery of growth hormone resulted in enlargement and enhanced proliferation of cutaneous lymphatic vessels in wild-type mice. These results identify growth hormone as a novel lymphangiogenic factor.
Figures


Similar articles
-
The specific effect of 2-methoxyestradiol on lymphatic vascular endothelial cells.Pharmazie. 2009 Mar;64(3):214-6. Pharmazie. 2009. PMID: 19348346
-
The effects of interleukin-7 on the lymphangiogenic properties of human endothelial cells.Int J Oncol. 2005 Sep;27(3):721-30. Int J Oncol. 2005. PMID: 16077922
-
Activation of the epidermal growth factor receptor promotes lymphangiogenesis in the skin.J Dermatol Sci. 2013 Sep;71(3):184-94. doi: 10.1016/j.jdermsci.2013.04.024. Epub 2013 May 4. J Dermatol Sci. 2013. PMID: 23706492 Free PMC article.
-
Regulation of endothelial cell differentiation and specification.Circ Res. 2013 Apr 26;112(9):1272-87. doi: 10.1161/CIRCRESAHA.113.300506. Circ Res. 2013. PMID: 23620236 Free PMC article. Review.
-
Molecular mechanisms of lymphangiogenesis in development and cancer.Int J Dev Biol. 2011;55(4-5):483-94. doi: 10.1387/ijdb.103226ia. Int J Dev Biol. 2011. PMID: 21858772 Review.
Cited by
-
Is there a risk of pubertal worsening in primary intestinal lymphangiectasia?J Endocrinol Invest. 2013 Dec;36(11):1128. doi: 10.3275/8974. Epub 2013 May 22. J Endocrinol Invest. 2013. PMID: 23698711 No abstract available.
-
Developmental and pathological lymphangiogenesis: from models to human disease.Histochem Cell Biol. 2008 Dec;130(6):1063-78. doi: 10.1007/s00418-008-0525-5. Epub 2008 Oct 23. Histochem Cell Biol. 2008. PMID: 18946678 Review.
-
Alternative splicing of leptin receptor overlapping transcript in osteosarcoma.Exp Biol Med (Maywood). 2020 Oct;245(16):1437-1443. doi: 10.1177/1535370220949139. Epub 2020 Aug 12. Exp Biol Med (Maywood). 2020. PMID: 32787464 Free PMC article.
-
Broad targeting of angiogenesis for cancer prevention and therapy.Semin Cancer Biol. 2015 Dec;35 Suppl(Suppl):S224-S243. doi: 10.1016/j.semcancer.2015.01.001. Epub 2015 Jan 16. Semin Cancer Biol. 2015. PMID: 25600295 Free PMC article. Review.
-
An in vivo method to quantify lymphangiogenesis in zebrafish.PLoS One. 2012;7(9):e45240. doi: 10.1371/journal.pone.0045240. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028871 Free PMC article.
References
-
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. - PubMed
-
- Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. - PubMed
-
- Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–1242. - PubMed
-
- Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. - PubMed
-
- Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Harkonen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98:946–951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

