The role of Rac2 in regulating neutrophil production in the bone marrow and circulating neutrophil counts
- PMID: 18583316
- PMCID: PMC2475787
- DOI: 10.2353/ajpath.2008.071059
The role of Rac2 in regulating neutrophil production in the bone marrow and circulating neutrophil counts
Abstract
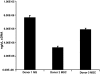
Circulating neutrophils are persistently higher in mice deficient in the small GTPase Rac2 than in wild-type (WT) mice. Therefore, we examined the mechanisms through which the small GTPase Rac2 regulates neutrophil production and release. Lethally irradiated WT mice reconstituted with a 50:50 mixture of WT and Rac2(-/-) fetal liver cells were protected from neutrophilia, suggesting that neutrophilia is primarily because of extrinsic defects that can be corrected by WT leukocytes. However, the differential counts and numbers of leukocyte subtypes differed between Rac2(-/-) and WT cells, suggesting that Rac2 modulates leukocyte lineage distribution. Kinetic studies suggest Rac2 modulates the release of neutrophils into the circulation and does not prolong their circulating half life. The percentage of bone marrow cells that expressed the neutrophil marker Gr-1 in lethally irradiated WT or Rac2(-/-) recipients of Rac2(-/-) stem cells was greater than in recipients of WT stem cells; however, circulating neutrophil counts were higher only in Rac2(-/-) recipients of Rac2(-/-) stem cells. Rac2 mRNA was expressed in the bone marrow of WT recipients of Rac2(-/-) stem cells and in human mesenchymal stem cells. The data presented here suggest that Rac2 in hematopoietic cells regulates leukocyte lineage distribution and Rac2 in nonhematopoietic cells might contribute to regulating circulating neutrophil counts.
Figures



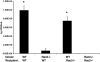
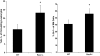
References
-
- Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. - PubMed
-
- Haataja L, Groffen J, Heisterkamp N. Characterization of RAC3, a novel member of the Rho family. J Biol Chem. 1997;272:20384–20388. - PubMed
-
- Gu Y, Jia B, Yang FC, D'Souza M, Harris CE, Derrow CW, Zheng Y, Williams DA. Biochemical and biological characterization of a human Rac2 GTPase mutant associated with phagocytic immunodeficiency. J Biol Chem. 2001;276:15929–15938. - PubMed
-
- Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, Levine JE, Petryniak B, Derrow CW, Harris C, Jia B, Zheng Y, Ambruso DR, Lowe JB, Atkinson SJ, Dinauer MC, Boxer L. Dominant negative mutation of the hematopoietic-specific Rho GTPase. Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

