Localization of PTP-1B, SHP-2, and Src exclusively in rat brain mitochondria and functional consequences
- PMID: 18583343
- PMCID: PMC3259839
- DOI: 10.1074/jbc.M709217200
Localization of PTP-1B, SHP-2, and Src exclusively in rat brain mitochondria and functional consequences
Abstract
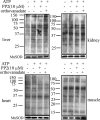
An immunodetection study of protein tyrosine phosphatase 1B (PTP-1B), SHP-2, and Src in isolated mitochondria from different rat tissues (brain, muscle, heart, liver, and kidney) revealed their exclusive localization in the brain. Given this result, we sought whether mitochondria respond to ATP and to the general tyrosine phosphatase inhibitor orthovanadate and found little or no change in the tyrosine phosphorylation profile of mitochondria from muscle, heart, liver, and kidney. In contrast, ATP induced an enhancement in the tyrosine-phosphorylated protein profile of brain mitochondria, which was further greatly enhanced with orthovanadate and which disappeared when Src was inhibited with two inhibitors: PP2 and PP1. Importantly, we found that in brain mitochondria, ATP addition induced Src autophosphorylation at Tyr-416 in its catalytic site, leading to its activation, whereas the regulatory Tyr-527 site remained unphosphorylated. Functional implications were addressed by measurements of the enzymatic activity of each of the oxidative phosphorylation complexes in brain mitochondria in the presence of ATP. We found an increase in complex I, III, and IV activity and a decrease in complex V activity, partially reversed by Src inhibition, demonstrating that the complexes are Src substrates. These results complemented and reinforced our initial study showing that respiration of brain mitochondria was partially dependent on tyrosine phosphorylation. Therefore, the present data suggest a possible control point in the regulation of respiration by tyrosine phosphorylation of the complexes mediated by Src auto-activation.
Figures




References
-
- Gnaiger, E., Lassnig, B., Kuznetsov, A., Rieger, G., and Margreiter, R. (1998) J. Exp. Biol. 201 1129–1139 - PubMed
-
- Devin, A., and Rigoulet, M. (2007) Am. J. Physiol. 292 C52–C58 - PubMed
-
- Benard, G., Faustin, B., Galinier, A., Rocher, C., Bellance, N., Smolkova, K., Casteilla, L., Rossignol, R., and Letellier, T. (2008) Int. J. Biochem. Cell Biol. 40 1545–1554 - PubMed
-
- Hüttemann, M., Lee, I., Samavati, L., Yu, H., and Doan, J. W. (2007) Biochim. Biophys. Acta 1773 1701–1720 - PubMed
-
- Pagliarini, D. J., and Dixon, J. E. (2006) Trends Biochem. Sci. 31 26–34 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

