The basis for selective E1-E2 interactions in the ISG15 conjugation system
- PMID: 18583345
- PMCID: PMC2527107
- DOI: 10.1074/jbc.M804069200
The basis for selective E1-E2 interactions in the ISG15 conjugation system
Abstract
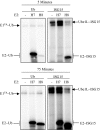
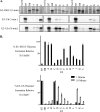
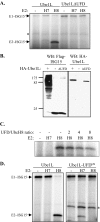
E1 and E2 enzymes coordinate the first steps in conjugation of ubiquitin (Ub) and ubiquitin-like proteins (Ubls). ISG15 is an interferon-alpha/beta-induced Ubl, and the E1 and E2 enzymes for ISG15 conjugation are Ube1L and UbcH8, respectively. UbcH7 is the most closely related E2 to UbcH8, yet it does not function in ISG15 conjugation in vivo, while both UbcH7 and UbcH8 have been reported to function in Ub conjugation. Kinetic analyses of wild-type and chimeric E2s were performed to determine the basis for preferential activation of UbcH8 by Ube1L and to determine whether UbcH8 is activated equally well by Ube1L and E1(Ub) (Ube1). K(m) determinations confirmed the strong preference of Ube1L for UbcH8 over UbcH7 (a 29-fold K(m) difference), similar to the preference of E1(Ub) for UbcH7 over UbcH8 (a 36-fold K(m) difference). Thioester assays of chimeric E2s identified two structural elements within residues 1-39 of UbcH8 that play a major role in defining Ube1L-UbcH8 specificity: the alpha1-helix and the beta1-beta2 region. The C-terminal ubiquitin fold domain (UFD) of Ube1L was required for transfer of ISG15 to UbcH8 and for binding of Ube1L to UbcH8. Replacement of the Ube1L UFD with that from E1(Ub) resulted in preferential transfer of ISG15 to UbcH7. Together, these results indicate that Ube1L discriminates between UbcH8 and closely related Ub E2s based on specific interactions between the Ube1L UFD and determinants within the N-terminal region of UbcH8.
Figures


References
-
- Pickart, C. M. (2001) Annu. Rev. Biochem. 70503 -533 - PubMed
-
- Melchior, F. (2000) Annu. Rev. Cell Dev. Biol. 16591 -626 - PubMed
-
- Scheffner, M., Nuber, U., and Huibregtse, J. M. (1995) Nature 37381 -83 - PubMed
-
- Kerscher, O., Felberbaum, R., and Hochstrasser, M. (2006) Annu. Rev. Cell Dev. Biol. 22 159-180 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

