Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function
- PMID: 18583454
- PMCID: PMC2847502
- DOI: 10.1124/mol.108.048843
Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function
Abstract
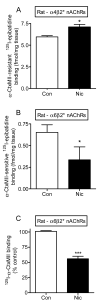
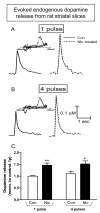
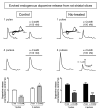
Nicotine treatment has long been associated with alterations in alpha4beta2(*) nicotinic acetylcholine receptor (nAChR) expression that modify dopaminergic function. However, the influence of long-term nicotine treatment on the alpha6beta2(*) nAChR, a subtype specifically localized on dopaminergic neurons, is less clear. Here we used voltammetry, as well as receptor binding studies, to identify the effects of nicotine on striatal alpha6beta2(*) nAChR function and expression. Long-term nicotine treatment via drinking water enhanced nonburst and burst endogenous dopamine release from rat striatal slices. In control animals, alpha6beta2(*) nAChR blockade with alpha-conotoxin MII (alpha-CtxMII) decreased release with nonburst stimulation but not with burst firing. These data in control animals suggest that varying stimulus frequencies differentially regulate alpha6beta2(*) nAChR-evoked dopamine release. In contrast, in nicotine-treated rats, alpha6beta2(*) nAChR blockade elicited a similar pattern of dopamine release with nonburst and burst firing. To elucidate the alpha6beta2(*) nAChR subtypes altered with long-term nicotine treatment, we used the novel alpha-CtxMII analog E11A in combination with alpha4 nAChR knockout mice. (125)I-alpha-CtxMII competition studies in striatum of knockout mice showed that nicotine treatment decreased the alpha6alpha4beta2(*) subtype but increased the alpha6(nonalpha4)beta2(*) nAChR population. These data indicate that alpha6beta2(*) nAChR-evoked dopamine release in nicotine-treated rats is mediated by the alpha6(nonalpha4)beta2(*) nAChR subtype and suggest that the alpha6alpha4beta2(*) nAChR and/or alpha4beta2(*) nAChR contribute to the differential effect of higher frequency stimulation on dopamine release under control conditions. Thus, alpha6beta2(*) nAChR subtypes may represent important targets for smoking cessation therapies and neurological disorders involving these receptors such as Parkinson's disease.
Figures



Similar articles
-
α6β2*-subtype nicotinic acetylcholine receptors are more sensitive than α4β2*-subtype receptors to regulation by chronic nicotine administration.J Neurochem. 2014 Jul;130(2):185-98. doi: 10.1111/jnc.12721. Epub 2014 Apr 19. J Neurochem. 2014. PMID: 24661093 Free PMC article.
-
Characterization of AN6001, a positive allosteric modulator of α6β2-containing nicotinic acetylcholine receptors.Biochem Pharmacol. 2020 Apr;174:113788. doi: 10.1016/j.bcp.2019.113788. Epub 2019 Dec 27. Biochem Pharmacol. 2020. PMID: 31887290
-
Long-term nicotine treatment down-regulates α6β2* nicotinic receptor expression and function in nucleus accumbens.J Neurochem. 2013 Dec;127(6):762-71. doi: 10.1111/jnc.12442. Epub 2013 Oct 13. J Neurochem. 2013. PMID: 23992036 Free PMC article.
-
Nicotinic receptor antagonists as treatments for nicotine abuse.Adv Pharmacol. 2014;69:513-51. doi: 10.1016/B978-0-12-420118-7.00013-5. Adv Pharmacol. 2014. PMID: 24484986 Free PMC article. Review.
-
The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum.Biochem Pharmacol. 2007 Oct 15;74(8):1235-46. doi: 10.1016/j.bcp.2007.07.032. Epub 2007 Jul 27. Biochem Pharmacol. 2007. PMID: 17825262 Free PMC article. Review.
Cited by
-
Common and separable neural alterations in substance use disorders: A coordinate-based meta-analyses of functional neuroimaging studies in humans.Hum Brain Mapp. 2020 Nov;41(16):4459-4477. doi: 10.1002/hbm.25085. Epub 2020 Sep 10. Hum Brain Mapp. 2020. PMID: 32964613 Free PMC article.
-
CHRNA5 gene variation affects the response of VTA dopaminergic neurons during chronic nicotine exposure and withdrawal.Neuropharmacology. 2023 Sep 1;235:109547. doi: 10.1016/j.neuropharm.2023.109547. Epub 2023 Apr 27. Neuropharmacology. 2023. PMID: 37116611 Free PMC article.
-
α6β2* and α4β2* nicotinic acetylcholine receptors as drug targets for Parkinson's disease.Pharmacol Rev. 2011 Dec;63(4):938-66. doi: 10.1124/pr.110.003269. Pharmacol Rev. 2011. PMID: 21969327 Free PMC article. Review.
-
Chronic treatment with varenicline changes expression of four nAChR binding sites in mice.Neuropharmacology. 2015 Dec;99:142-55. doi: 10.1016/j.neuropharm.2015.07.019. Epub 2015 Jul 17. Neuropharmacology. 2015. PMID: 26192545 Free PMC article.
-
Neuronal Excitability in the Medial Habenula and Ventral Tegmental Area Is Differentially Modulated by Nicotine Dosage and Menthol in a Sex-Specific Manner.eNeuro. 2024 Feb 12;11(2):ENEURO.0380-23.2024. doi: 10.1523/ENEURO.0380-23.2024. Print 2024 Feb. eNeuro. 2024. PMID: 38233142 Free PMC article.
References
-
- Bordia T, Grady SR, McIntosh JM, Quik M. Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson's disease striatum. Mol Pharmacol. 2007;72(1):52–61. - PubMed
-
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. - PubMed
-
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74(8):1102–1111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA12242/DA/NIDA NIH HHS/United States
- R01 DA003194/DA/NIDA NIH HHS/United States
- R01 DA012242/DA/NIDA NIH HHS/United States
- P01 GM048677/GM/NIGMS NIH HHS/United States
- R01 NS059910/NS/NINDS NIH HHS/United States
- MH53631/MH/NIMH NIH HHS/United States
- R01 NS042091/NS/NINDS NIH HHS/United States
- DA03194/DA/NIDA NIH HHS/United States
- R01 NS047162/NS/NINDS NIH HHS/United States
- NS42091/NS/NINDS NIH HHS/United States
- GM48677/GM/NIGMS NIH HHS/United States
- R01 MH053631/MH/NIMH NIH HHS/United States
- NS47162/NS/NINDS NIH HHS/United States
- R29 MH053631/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

