Activation of the A(3) adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils
- PMID: 18583455
- PMCID: PMC2574951
- DOI: 10.1124/mol.108.048066
Activation of the A(3) adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils
Abstract
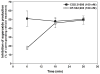
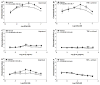
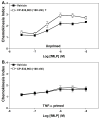
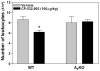
Adenosine is formed in injured/ischemic tissues, where it suppresses the actions of essentially all cells of the immune system. Most of the anti-inflammatory actions of adenosine have been attributed to signaling through the G(s) protein-coupled A(2A) adenosine receptor (AR). Here, we report that the A(3)AR is highly expressed in murine neutrophils isolated from bone marrow. Selective activation of the A(3)AR with (2S,3S,4R,5R)-3-amino-5-[6-(2,5-dichlorobenzylamino)purin-9-yl]-4-hydroxytetrahydrofuran-2-carboxylic acid methylamide (CP-532,903) potently inhibited mouse bone marrow neutrophil superoxide generation and chemotaxis induced by various activating agents. The selectivity of CP-532,903 was confirmed in assays using neutrophils obtained from A(2A)AR and A(3)AR gene "knockout" mice. In a model of thioglycollate-induced inflammation, treating mice with CP-532,903 inhibited recruitment of leukocytes into the peritoneum by specifically activating the A(3)AR. Collectively, our findings support the theory that the A(3)AR contributes to the anti-inflammatory actions of adenosine on neutrophils and provide a potential mechanistic explanation for the efficacy of A(3)AR agonists in animal models of inflammation (i.e., inhibition of neutrophil-mediated tissue injury).
Figures








References
-
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. - PubMed
-
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. - PubMed
-
- Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

