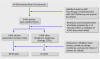
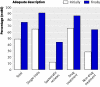
What is missing from descriptions of treatment in trials and reviews?
- PMID: 18583680
- PMCID: PMC2440840
- DOI: 10.1136/bmj.39590.732037.47
What is missing from descriptions of treatment in trials and reviews?
Abstract
Replicating non-pharmacological treatments in practice depends on how well they have been described in research studies
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust 2006;185:263-7. - PubMed
-
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-65. - PubMed
-
- Dopson S, Locock L, Chambers D, Gabbay J. Implementation of evidence-based medicine: evaluation of the promoting action on clinical effectiveness programme. J Health Serv Res Policy 2006;6:23-31. - PubMed
-
- Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. The case of pediatric vaccine recommendations. Med Care 1996;34:873-89. - PubMed
-
- Glasziou P, Haynes B. The paths from research to improved health outcomes. ACP J Club 2005;142:A8-10. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical