Localized alpha4 integrin phosphorylation directs shear stress-induced endothelial cell alignment
- PMID: 18583710
- PMCID: PMC2669779
- DOI: 10.1161/CIRCRESAHA.108.176354
Localized alpha4 integrin phosphorylation directs shear stress-induced endothelial cell alignment
Abstract
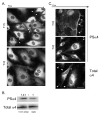
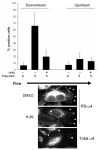
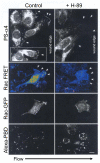
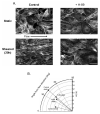
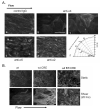
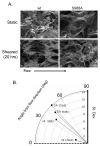
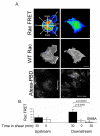
Vascular endothelial cells respond to laminar shear stress by aligning in the direction of flow, a process which may contribute to atheroprotection. Here we report that localized alpha4 integrin phosphorylation is a mechanism for establishing the directionality of shear stress-induced alignment in microvascular endothelial cells. Within 5 minutes of exposure to a physiological level of shear stress, endothelial alpha4 integrins became phosphorylated on Ser(988). In wounded monolayers, phosphorylation was enhanced at the downstream edges of cells relative to the source of flow. The shear-induced alpha4 integrin phosphorylation was blocked by inhibitors of cAMP-dependent protein kinase A (PKA), an enzyme involved in the alignment of endothelial cells under prolonged shear. Moreover, shear-induced localized activation of the small GTPase Rac1, which specifies the directionality of endothelial alignment, was similarly blocked by PKA inhibitors. Furthermore, endothelial cells bearing a nonphosphorylatable alpha4(S(988)A) mutation failed to align in response to shear stress, thus establishing alpha4 as a relevant PKA substrate. We thereby show that shear-induced PKA-dependent alpha4 integrin phosphorylation at the downstream edge of endothelial cells promotes localized Rac1 activation, which in turn directs cytoskeletal alignment in response to shear stress.
Figures

Comment in
-
Choosing sides in polarized endothelial adaptation to shear stress.Circ Res. 2008 Jul 18;103(2):122-4. doi: 10.1161/CIRCRESAHA.108.180836. Circ Res. 2008. PMID: 18635827 Free PMC article. No abstract available.
Similar articles
-
Choosing sides in polarized endothelial adaptation to shear stress.Circ Res. 2008 Jul 18;103(2):122-4. doi: 10.1161/CIRCRESAHA.108.180836. Circ Res. 2008. PMID: 18635827 Free PMC article. No abstract available.
-
Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization.Am J Physiol Heart Circ Physiol. 2005 Feb;288(2):H936-45. doi: 10.1152/ajpheart.00519.2004. Epub 2004 Oct 7. Am J Physiol Heart Circ Physiol. 2005. PMID: 15471980
-
Alpha4 integrins are type I cAMP-dependent protein kinase-anchoring proteins.Nat Cell Biol. 2007 Apr;9(4):415-21. doi: 10.1038/ncb1561. Epub 2007 Mar 18. Nat Cell Biol. 2007. PMID: 17369818
-
The role of the alpha4 integrin-paxillin interaction in regulating leukocyte trafficking.Exp Mol Med. 2006 Jun 30;38(3):191-5. doi: 10.1038/emm.2006.23. Exp Mol Med. 2006. PMID: 16819276 Review.
-
Rac[e] to the pole: setting up polarity in endothelial cells.Small GTPases. 2014;5:e28650. doi: 10.4161/sgtp.28650. Epub 2014 Mar 31. Small GTPases. 2014. PMID: 25202973 Free PMC article. Review.
Cited by
-
Protein kinase A activity is regulated by actomyosin contractility during cell migration and is required for durotaxis.Mol Biol Cell. 2020 Jan 1;31(1):45-58. doi: 10.1091/mbc.E19-03-0131. Epub 2019 Nov 13. Mol Biol Cell. 2020. PMID: 31721649 Free PMC article.
-
VLA-4 phosphorylation during tumor and immune cell migration relies on its coupling to VEGFR2 and CXCR4 by syndecan-1.J Cell Sci. 2019 Oct 28;132(20):jcs232645. doi: 10.1242/jcs.232645. J Cell Sci. 2019. PMID: 31562188 Free PMC article.
-
Matrix-specific protein kinase A signaling regulates p21-activated kinase activation by flow in endothelial cells.Circ Res. 2010 Apr 30;106(8):1394-403. doi: 10.1161/CIRCRESAHA.109.210286. Epub 2010 Mar 11. Circ Res. 2010. PMID: 20224042 Free PMC article.
-
Syndecans and Their Synstatins: Targeting an Organizer of Receptor Tyrosine Kinase Signaling at the Cell-Matrix Interface.Front Oncol. 2021 Oct 27;11:775349. doi: 10.3389/fonc.2021.775349. eCollection 2021. Front Oncol. 2021. PMID: 34778093 Free PMC article. Review.
-
PDGF-BB and TGF-{beta}1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress.Proc Natl Acad Sci U S A. 2011 Feb 1;108(5):1908-13. doi: 10.1073/pnas.1019219108. Epub 2011 Jan 18. Proc Natl Acad Sci U S A. 2011. PMID: 21245329 Free PMC article.
References
-
- Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999 September 17;85(6):504–14. - PubMed
-
- Gojova A, Barakat AI. Vascular endothelial wound closure under shear stress: role of membrane fluidity and flow-sensitive ion channels. J Appl Physiol. 2005 June;98(6):2355–62. - PubMed
-
- Hsu PP, Li S, Li YS, Usami S, Ratcliffe A, Wang X, Chien S. Effects of flow patterns on endothelial cell migration into a zone of mechanical denudation. Biochem Biophys Res Commun. 2001 July 20;285(3):751–9. - PubMed
-
- Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005 January;85(1):9–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL085159/HL/NHLBI NIH HHS/United States
- GM68524/GM/NIGMS NIH HHS/United States
- K12 GM068524/GM/NIGMS NIH HHS/United States
- HL31950/HL/NHLBI NIH HHS/United States
- R01 AR027214/AR/NIAMS NIH HHS/United States
- R01 HL088632/HL/NHLBI NIH HHS/United States
- P01 HL031950/HL/NHLBI NIH HHS/United States
- P01 HL078784/HL/NHLBI NIH HHS/United States
- HL078784/HL/NHLBI NIH HHS/United States
- AR27214/AR/NIAMS NIH HHS/United States
- HL085159/HL/NHLBI NIH HHS/United States
- R37 AR027214/AR/NIAMS NIH HHS/United States
- HL088632/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

