Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia
- PMID: 18583711
- PMCID: PMC2711765
- DOI: 10.1161/CIRCRESAHA.108.176230
Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia
Abstract
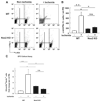
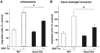
Bone marrow (BM) is the major reservoir for endothelial progenitor cells (EPCs). Postnatal neovascularization depends on not only angiogenesis but also vasculogenesis, which is mediated through mobilization of EPCs from BM and their recruitment to the ischemic sites. Reactive oxygen species (ROS) derived from Nox2-based NADPH oxidase play an important role in postnatal neovascularization; however, their role in BM and EPC function is unknown. Here we show that hindlimb ischemia of mice significantly increases Nox2 expression and ROS production in BM-mononuclear cells (BMCs), which is associated with an increase in circulating EPC-like cells. Mice lacking Nox2 show reduction of ischemia-induced flow recovery, ROS levels in BMCs, as well as EPC mobilization from BM. Transplantation of wild-type (WT)-BM into Nox2-deficient mice rescues the defective neovascularization, whereas WT mice transplanted with Nox2-deficient BM show reduced flow recovery and capillary density compared to WT-BM transplanted control. Intravenous infusion of WT- and Nox2-deficient BMCs into WT mice reveals that neovascularization and homing capacity are impaired in Nox2-deficient BMCs in vivo. In vitro, Nox2-deficient c-kit+Lin- BM stem/progenitor cells show impaired chemotaxis and invasion as well as polarization of actins in response to stromal derived factor (SDF), which is associated with blunted SDF-1-mediated phosphorylation of Akt. In conclusion, Nox2-derived ROS in BM play a critical role in mobilization, homing, and angiogenic capacity of EPCs and BM stem/progenitor cells, thereby promoting revascularization of ischemic tissue. Thus, NADPH oxidase in BM and EPCs is potential therapeutic targets for promoting neovascularization in ischemic cardiovascular diseases.
Figures
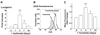

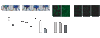



References
-
- Isner JM, Losordo DW. Therapeutic angiogenesis for heart failure. Nat Med. 1999;5:491–492. - PubMed
-
- Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. - PubMed
-
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. - PubMed
-
- Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. - PubMed
-
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exper Hematol. 2002;30:973–981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

