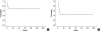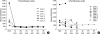Treatment outcomes with CHOP chemotherapy in adult patients with hemophagocytic lymphohistiocytosis
- PMID: 18583880
- PMCID: PMC2526520
- DOI: 10.3346/jkms.2008.23.3.439
Treatment outcomes with CHOP chemotherapy in adult patients with hemophagocytic lymphohistiocytosis
Abstract
The objective of the current study was to investigate the treatment outcomes for the use of cyclophosphamide, adriamycin, vincristine, and prednisolone (CHOP) chemotherapy in adult patients with hemophagocytic lymphohistiocytosis (HLH). Seventeen HLH patients older than 18 yr of age were treated with CHOP chemotherapy. A response evaluation was conducted for every two cycles of chemotherapy. With CHOP chemotherapy, complete response was achieved for 7/17 patients (41.2%), a partial response for 3/17 patients (17.6%), and the overall response rate was 58.8%. The median response duration (RD) was not reached and the 2-yr RD rate was 68.6%, with a median follow-up of 100 weeks. Median overall survival (OS) was 18 weeks (95% CI, 6-30 weeks) and the 2-yr OS rate was 43.9%. Reported grade 3 or 4 non-hematological toxicities were increased serum liver enzyme levels and stomatitis. Grade 3 or 4 hematological toxicities were leukopenia (50.8%), anemia (20%), and thrombocytopenia (33.9%). Neutropenic fever was observed in 21.6% of patients (14/65 cycles), and most of the cases were resolved with supportive care including treatment with broad-spectrum antibiotics. CHOP chemotherapy seems to be effective in adult HLH patients and the toxicities are manageable.
Figures
References
-
- Janka GE, Schneider EM. Modern management of children with haemophagocytic lymphohistiocytosis. Br J Haematol. 2004;124:4–14. - PubMed
-
- Miyahara M, Sano M, Shibata K, Matsuzaki M, Ibaraki K, Shimamoto Y, Tokunaga O. B-cell lymphoma-associated hemophagocytic syndrome: clinicopathological characteristics. Ann Hematol. 2000;79:378–388. - PubMed
-
- Imashuku S, Hibi S, Ohara T, Iwai A, Sako M, Kato M, Arakawa H, Sotomatsu M, Kataoka S, Asami K, Hasegawa D, Kosaka Y, Sano K, Igarashi N, Maruhashi K, Ichimi R, Kawasaki H, Maeda N, Tanizawa A, Arai K, Abe T, Hisakawa H, Miyashita H, Henter JI. Effective control of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis with immunochemotherapy. Histiocyte Society. Blood. 1999;93:1869–1874. - PubMed
-
- Gencik A, Signer E, Muller H. Genetic analysis of familial erythrophagocytic lymphohistiocytosis. Eur J Pediatr. 1984;142:248–252. - PubMed
-
- Henter JI, Arico M, Egeler RM, Elinder G, Favara BE, Filipovich AH, Gadner H, Imashuku S, Janka-Schaub G, Komp D, Ladisch S, Webb D. HLH-94: a treatment protocol for hemophagocytic lymphohistiocytosis. HLH study group of the Histiocyte Society. Med Pediatr Oncol. 1997;28:342–347. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous