Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage
- PMID: 18583988
- PMCID: PMC2442910
- DOI: 10.1038/embor.2008.103
Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage
Abstract
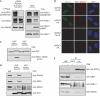
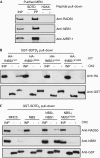
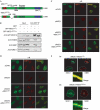
Mammalian cells respond to DNA double-strand breaks (DSBs) by recruiting DNA repair and cell-cycle checkpoint proteins to such sites. Central to these DNA damage response (DDR) events is the DNA damage mediator protein MDC1. MDC1 interacts with several DDR proteins, including the MRE11-RAD50-NBS1 (MRN) complex. Here, we show that MDC1 is phosphorylated on a cluster of conserved repeat motifs by casein kinase 2 (CK2). Moreover, we establish that this phosphorylation of MDC1 promotes direct, phosphorylation-dependent interactions with NBS1 in a manner that requires the closely apposed FHA and twin BRCT domains in the amino terminus of NBS1. Finally, we show that these CK2-targeted motifs in MDC1 are required to mediate NBS1 association with chromatin-flanking sites of unrepaired DSBs. These findings provide a molecular explanation for the MDC1-MRN interaction and yield insights into how MDC1 coordinates the focal assembly and activation of several DDR factors in response to DNA damage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Becker E, Meyer V, Madaoui H, Guerois R (2006) Detection of a tandem BRCT in Nbs1 and Xrs2 with functional implications in the DNA damage response. Bioinformatics 22: 1289–1292 - PubMed
-
- Clements PM, Breslin C, Deeks ED, Byrd PJ, Ju L, Bieganowski P, Brenner C, Moreira MC, Taylor AM, Caldecott KW (2004) The ataxia-oculomotor apraxia 1 gene product has a role distinct from ATM and interacts with the DNA strand break repair proteins XRCC1 and XRCC4. DNA Repair 3: 1493–1502 - PubMed
-
- Durocher D, Taylor IA, Sarbassova D, Haire LF, Westcott SL, Jackson SP, Smerdon SJ, Yaffe MB (2000) The molecular basis of FHA domain:phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol Cell 6: 1169–1182 - PubMed
-
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A (2004) H2AX: the histone guardian of the genome. DNA Repair 3: 959–967 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

