Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes
- PMID: 18584034
- PMCID: PMC2432031
- DOI: 10.1371/journal.pgen.1000116
Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes
Abstract
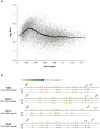
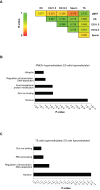
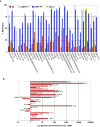
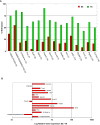
DNA methylation patterns are reprogrammed in primordial germ cells and in preimplantation embryos by demethylation and subsequent de novo methylation. It has been suggested that epigenetic reprogramming may be necessary for the embryonic genome to return to a pluripotent state. We have carried out a genome-wide promoter analysis of DNA methylation in mouse embryonic stem (ES) cells, embryonic germ (EG) cells, sperm, trophoblast stem (TS) cells, and primary embryonic fibroblasts (pMEFs). Global clustering analysis shows that methylation patterns of ES cells, EG cells, and sperm are surprisingly similar, suggesting that while the sperm is a highly specialized cell type, its promoter epigenome is already largely reprogrammed and resembles a pluripotent state. Comparisons between pluripotent tissues and pMEFs reveal that a number of pluripotency related genes, including Nanog, Lefty1 and Tdgf1, as well as the nucleosome remodeller Smarcd1, are hypomethylated in stem cells and hypermethylated in differentiated cells. Differences in promoter methylation are associated with significant differences in transcription levels in more than 60% of genes analysed. Our comparative approach to promoter methylation thus identifies gene candidates for the regulation of pluripotency and epigenetic reprogramming. While the sperm genome is, overall, similarly methylated to that of ES and EG cells, there are some key exceptions, including Nanog and Lefty1, that are highly methylated in sperm. Nanog promoter methylation is erased by active and passive demethylation after fertilisation before expression commences in the morula. In ES cells the normally active Nanog promoter is silenced when targeted by de novo methylation. Our study suggests that reprogramming of promoter methylation is one of the key determinants of the epigenetic regulation of pluripotency genes. Epigenetic reprogramming in the germline prior to fertilisation and the reprogramming of key pluripotency genes in the early embryo is thus crucial for transmission of pluripotency.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. - PubMed
-
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. - PubMed
-
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. - PubMed
-
- Schaefer CB, Ooi SKT, Bestor TH, Bourc'his D. Epigenetic Decisions in Mammalian Germ Cells. Science. 2007;316:398–399. - PubMed
-
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0700098/MRC_/Medical Research Council/United Kingdom
- G0800784/MRC_/Medical Research Council/United Kingdom
- BBS/E/B/0000M100/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0300723/MRC_/Medical Research Council/United Kingdom
- G120/824/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

