Synthesis of macrocyclic trypanosomal cysteine protease inhibitors
- PMID: 18585034
- PMCID: PMC2642929
- DOI: 10.1016/j.bmcl.2008.06.012
Synthesis of macrocyclic trypanosomal cysteine protease inhibitors
Abstract
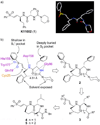
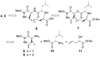
The importance of cysteine proteases in parasites, compounded with the lack of redundancy compared to their mammalian hosts makes proteases attractive targets for the development of new therapeutic agents. The binding mode of K11002 to cruzain, the major cysteine protease of Trypanosoma cruzi was used in the design of conformationally constrained inhibitors. Vinyl sulfone-containing macrocycles were synthesized via olefin ring-closing metathesis and evaluated against cruzain and the closely related cysteine protease, rhodesain.
Figures


Similar articles
-
Structure-based design, synthesis and evaluation of conformationally constrained cysteine protease inhibitors.Bioorg Med Chem. 1998 Dec;6(12):2477-94. doi: 10.1016/s0968-0896(98)80022-9. Bioorg Med Chem. 1998. PMID: 9925304
-
Design, synthesis and biological evaluation of potent azadipeptide nitrile inhibitors and activity-based probes as promising anti-Trypanosoma brucei agents.Chemistry. 2012 May 21;18(21):6528-41. doi: 10.1002/chem.201103322. Epub 2012 Apr 4. Chemistry. 2012. PMID: 22488888
-
Potent second generation vinyl sulfonamide inhibitors of the trypanosomal cysteine protease cruzain.Bioorg Med Chem Lett. 2001 Oct 22;11(20):2759-62. doi: 10.1016/s0960-894x(01)00566-2. Bioorg Med Chem Lett. 2001. PMID: 11591518
-
Synthesis and structure-activity relationship studies of cruzain and rhodesain inhibitors.Eur J Med Chem. 2018 Sep 5;157:1426-1459. doi: 10.1016/j.ejmech.2018.08.079. Epub 2018 Aug 31. Eur J Med Chem. 2018. PMID: 30282318 Review.
-
Cruzain : the path from target validation to the clinic.Adv Exp Med Biol. 2011;712:100-15. doi: 10.1007/978-1-4419-8414-2_7. Adv Exp Med Biol. 2011. PMID: 21660661 Review.
Cited by
-
Chloroaluminate Ionic Liquid Immobilized on Magnetic Nanoparticles as a Heterogeneous Lewis Acidic Catalyst for the Friedel-Crafts Sulfonylation of Aromatic Compounds.Molecules. 2022 Mar 2;27(5):1644. doi: 10.3390/molecules27051644. Molecules. 2022. PMID: 35268745 Free PMC article.
-
Chemical-biological characterization of a cruzain inhibitor reveals a second target and a mammalian off-target.Beilstein J Org Chem. 2013;9:15-25. doi: 10.3762/bjoc.9.3. Epub 2013 Jan 4. Beilstein J Org Chem. 2013. PMID: 23400640 Free PMC article.
-
Drug Discovery for Cutaneous Leishmaniasis: A Review of Developments in the Past 15 Years.Microorganisms. 2023 Nov 23;11(12):2845. doi: 10.3390/microorganisms11122845. Microorganisms. 2023. PMID: 38137989 Free PMC article. Review.
-
Predictive Global Models of Cruzain Inhibitors with Large Chemical Coverage.ACS Omega. 2021 Mar 5;6(10):6722-6735. doi: 10.1021/acsomega.0c05645. eCollection 2021 Mar 16. ACS Omega. 2021. PMID: 33748586 Free PMC article.
-
Two approaches to discovering and developing new drugs for Chagas disease.Mem Inst Oswaldo Cruz. 2009 Jul;104 Suppl 1(0 1):263-9. doi: 10.1590/s0074-02762009000900034. Mem Inst Oswaldo Cruz. 2009. PMID: 19753483 Free PMC article.
References
-
- Lancet. 2006;368:619. - PubMed
-
- World Health Organization. http://www.who.int/tdr/diseases/chagas/default.htm.
-
- World Health Organization. http://www.cdc.gov/chagas/factsheet.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

