Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma
- PMID: 18585064
- PMCID: PMC2601709
- DOI: 10.1016/j.immuni.2008.04.022
Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma
Abstract
gammadelta T cells uniquely contribute to host immune defense, but how this is accomplished remains unclear. Here, we analyzed the nonclassical major histocompatibility complex class I T10 and T22-specific gammadelta T cells in mice and found that encountering antigen in the thymus was neither required nor inhibitory for their development. But when triggered through the T cell receptor, ligand-naive lymphoid-gammadelta T cells produced IL-17, whereas ligand-experienced cells made IFN-gamma. Immediately after immunization, a large fraction of IL-17(+) gammadelta T cells were found in the draining lymph nodes days before the appearance of antigen-specific IL-17(+) *beta T cells. Thus, thymic selection determines the effector fate of gammadelta T cells rather than constrains their antigen specificities. The swift IL-17 response mounted by antigen-naive gammadelta T cells suggests a critical role for these cells at the onset of an acute inflammatory response to novel antigens.
Figures
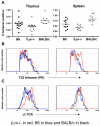





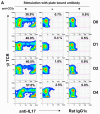


Comment in
-
gammadelta T cell selection: is anyone useless?Immunity. 2008 Jul 18;29(1):3-5. doi: 10.1016/j.immuni.2008.06.007. Immunity. 2008. PMID: 18631449
Similar articles
-
Thymic maturation determines gammadelta T cell function, but not their antigen specificities.Curr Opin Immunol. 2009 Apr;21(2):140-5. doi: 10.1016/j.coi.2009.02.008. Epub 2009 Mar 25. Curr Opin Immunol. 2009. PMID: 19321327 Free PMC article. Review.
-
Differentiation and activation of γδ T Lymphocytes: Focus on CD27 and CD28 costimulatory receptors.Adv Exp Med Biol. 2013;785:95-105. doi: 10.1007/978-1-4614-6217-0_11. Adv Exp Med Biol. 2013. PMID: 23456842 Review.
-
γδ T cells acquire effector fates in the thymus and differentiate into cytokine-producing effectors in a Listeria model of infection independently of CD28 costimulation.PLoS One. 2013 May 9;8(5):e63178. doi: 10.1371/journal.pone.0063178. Print 2013. PLoS One. 2013. PMID: 23671671 Free PMC article.
-
Ligand recognition during thymic development and gammadelta T cell function specification.Semin Immunol. 2010 Aug;22(4):207-13. doi: 10.1016/j.smim.2010.04.002. Epub 2010 Apr 28. Semin Immunol. 2010. PMID: 20430644 Free PMC article. Review.
-
Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers.J Immunol. 2008 Nov 1;181(9):5940-7. doi: 10.4049/jimmunol.181.9.5940. J Immunol. 2008. PMID: 18941182
Cited by
-
Noncanonical mode of ERK action controls alternative αβ and γδ T cell lineage fates.Immunity. 2014 Dec 18;41(6):934-46. doi: 10.1016/j.immuni.2014.10.021. Epub 2014 Nov 28. Immunity. 2014. PMID: 25526308 Free PMC article.
-
The divergence between T cell and innate lymphoid cell fates controlled by E and Id proteins.Front Immunol. 2022 Aug 10;13:960444. doi: 10.3389/fimmu.2022.960444. eCollection 2022. Front Immunol. 2022. PMID: 36032069 Free PMC article. Review.
-
Mechanisms underlying lineage commitment and plasticity of human γδ T cells.Cell Mol Immunol. 2013 Jan;10(1):30-4. doi: 10.1038/cmi.2012.42. Epub 2012 Oct 22. Cell Mol Immunol. 2013. PMID: 23085943 Free PMC article. Review.
-
γδ T cell receptors recognize the non-classical major histocompatibility complex (MHC) molecule T22 via conserved anchor residues in a MHC peptide-like fashion.J Biol Chem. 2012 Feb 17;287(8):6035-43. doi: 10.1074/jbc.M111.333153. Epub 2012 Jan 3. J Biol Chem. 2012. PMID: 22215668 Free PMC article.
-
αβ versus γδ fate choice: counting the T-cell lineages at the branch point.Immunol Rev. 2010 Nov;238(1):169-81. doi: 10.1111/j.1600-065X.2010.00947.x. Immunol Rev. 2010. PMID: 20969592 Free PMC article. Review.
References
-
- Adams EJ, Chien YH, Garcia KC. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. - PubMed
-
- Baccala R, Witherden D, Gonzalez-Quintial R, Dummer W, Surh CD, Havran WL, Theofilopoulos AN. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. 2005;174:4606–4612. - PubMed
-
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. - PubMed
-
- Bonneville M, Ishida I, Itohara S, Verbeek S, Berns A, Kanagawa O, Haas W, Tonegawa S. Self-tolerance to transgenic gamma delta T cells by intrathymic inactivation. Nature. 1990;344:163–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

