Molecular antagonism and plasticity of regulatory and inflammatory T cell programs
- PMID: 18585065
- PMCID: PMC2630532
- DOI: 10.1016/j.immuni.2008.05.007
Molecular antagonism and plasticity of regulatory and inflammatory T cell programs
Abstract
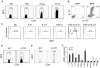
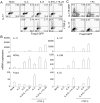
Regulatory T (Treg) and T helper 17 (Th17) cells were recently proposed to be reciprocally regulated during differentiation. To understand the underlying mechanisms, we utilized a Th17 reporter mouse with a red fluorescent protein (RFP) sequence inserted into the interleukin-17F (IL-17F) gene. Using IL-17F-RFP together with a Foxp3 reporter, we found that the development of Th17 and Foxp3(+) Treg cells was associated in immune responses. Although TGF-beta receptor I signaling was required for both Foxp3 and IL-17 induction, SMAD4 was only involved in Foxp3 upregulation. Foxp3 inhibited Th17 differentiation by antagonizing the function of the transcription factors RORgammat and ROR*. In contrast, IL-6 overcame this suppressive effect of Foxp3 and, together with IL-1, induced genetic reprogramming in Foxp3(+) Treg cells. STAT3 regulated Foxp3 downregulation, whereas STAT3, RORgamma, and ROR* were required for IL-17 expression in Treg cells. Our data demonstrate molecular antagonism and plasticity of Treg and Th17 cell programs.
Figures





Comment in
-
T-regulatory/T-helper 17: promiscuous signals and fear of commitment.Immunotherapy. 2009 Jan;1(1):27-9. doi: 10.2217/1750743X.1.1.27. Immunotherapy. 2009. PMID: 20635970
References
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
-
- Chu GC, Dunn NR, Anderson DC, Oxburgh L, Robertson EJ. Differential requirements for Smad4 in TGF{beta}-dependent patterning of the early mouse embryo. Development. 2004;131:3501–3512. - PubMed
-
- Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. - PubMed
-
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

