Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis
- PMID: 18585375
- PMCID: PMC2582391
- DOI: 10.1016/j.ydbio.2008.06.005
Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis
Abstract
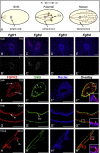
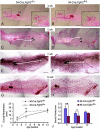
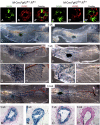
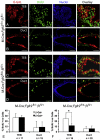
FGF signaling is associated with breast cancer and is required for mammary placode formation in the mouse. In this study, we employed a genetic mosaic analysis based on Cre-mediated recombination to investigate FGF receptor 2 (Fgfr2) function in the postnatal mammary gland. Mosaic inactivation of Fgfr2 by the MMTV-Cre transgene enabled us to compare the behavior of Fgfr2 null and Fgfr2 heterozygous cells in the same gland. Fgfr2 null cells were at a competitive disadvantage to their Fgfr2 heterozygous neighbors in the highly proliferative terminal end buds (TEBs) at the invasion front, owing to a negative effect of loss of Fgfr2 function on cell proliferation. However, Fgfr2 null cells were tolerated in mature ducts. In these genetic mosaic mammary glands, the epithelial network is apparently built by TEBs that over time are composed of a progressively larger proportion of Fgfr2-positive cells. However, subsequently, most cells lose Fgfr2 function, presumably due to additional rounds of Cre-mediated recombination. Using an independent strategy to create mosaic mammary glands, which employed an adenovirus-Cre that acts only once, we confirmed that Fgfr2 null cells were out-competed by neighboring Fgfr2 heterozygous cells. Together, our data demonstrate that Fgfr2 functions in the proliferating and invading TEBs, but it is not required in the mature ducts of the pubertal mammary gland.
Figures


References
-
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell. 2003;4:11–18. - PubMed
-
- Beer HD, Florence C, Dammeier J, McGuire L, Werner S, Duan DR. Mouse fibroblast growth factor 10: cDNA cloning, protein characterization, and regulation of mRNA expression. Oncogene. 1997;15:2211–2218. - PubMed
-
- Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 2001;142:4937–4945. - PubMed
-
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005;26:63–77. - PubMed
-
- Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev. Cell. 2005;9:831–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

