Heterodimerization with small Maf proteins enhances nuclear retention of Nrf2 via masking the NESzip motif
- PMID: 18585411
- PMCID: PMC2570698
- DOI: 10.1016/j.bbamcr.2008.05.024
Heterodimerization with small Maf proteins enhances nuclear retention of Nrf2 via masking the NESzip motif
Abstract
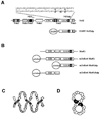
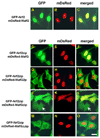
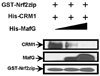
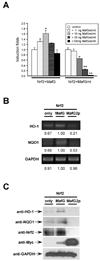
Nrf2 is the key transcription factor regulating the antioxidant response. When exposed to oxidative stress, Nrf2 translocates to cell nucleus and forms heterodimer with small Maf proteins (sMaf). Nrf2/sMaf heterodimer binds specifically to a cis-acting enhancer called antioxidant response element and initiates transcription of a battery of antioxidant and detoxification genes. Nrf2 possesses a NESzip motif (nuclear export signal co-localized with the leucine zipper (ZIP) domain). Heterodimerization with MafG via ZIP-ZIP binding enhanced Nrf2 nuclear retention, which could be abrogated by the deletion of the ZIP domain or site-directed mutations targeting at the ZIP domain. In addition, dimerization with MafG precluded Nrf2zip/CRM1 binding, suggesting that Nrf2/MafG heterodimerization may simultaneously mask the NESzip motif. MafG-mediated nuclear retention may enable Nrf2 proteins to evade cytosolic proteasomal degradation and consequently stabilize Nrf2 signaling. For the first time, we show that under the physiological condition, the NESzip motif can be switched-off by heterodimerization.
Figures

Similar articles
-
Nrf2 Possesses a redox-insensitive nuclear export signal overlapping with the leucine zipper motif.J Biol Chem. 2005 Aug 5;280(31):28430-8. doi: 10.1074/jbc.M410601200. Epub 2005 May 23. J Biol Chem. 2005. PMID: 15917227
-
Molecular determinants for small Maf protein control of platelet production.Mol Cell Biol. 2011 Jan;31(1):151-62. doi: 10.1128/MCB.00798-10. Epub 2010 Oct 25. Mol Cell Biol. 2011. PMID: 20974807 Free PMC article.
-
Molecular basis distinguishing the DNA binding profile of Nrf2-Maf heterodimer from that of Maf homodimer.J Biol Chem. 2007 Nov 16;282(46):33681-33690. doi: 10.1074/jbc.M706863200. Epub 2007 Sep 17. J Biol Chem. 2007. PMID: 17875642
-
Cis-element architecture of Nrf2-sMaf heterodimer binding sites and its relation to diseases.Arch Pharm Res. 2020 Mar;43(3):275-285. doi: 10.1007/s12272-019-01193-2. Epub 2019 Dec 2. Arch Pharm Res. 2020. PMID: 31792803 Review.
-
Discovery of the negative regulator of Nrf2, Keap1: a historical overview.Antioxid Redox Signal. 2010 Dec 1;13(11):1665-78. doi: 10.1089/ars.2010.3222. Epub 2010 Jul 13. Antioxid Redox Signal. 2010. PMID: 20446768 Review.
Cited by
-
Inhibition of the Nrf2/HO-1 Axis Suppresses the Mitochondria-Related Protection Promoted by Gastrodin in Human Neuroblastoma Cells Exposed to Paraquat.Mol Neurobiol. 2019 Mar;56(3):2174-2184. doi: 10.1007/s12035-018-1222-6. Epub 2018 Jul 11. Mol Neurobiol. 2019. PMID: 29998398
-
Structure-activity relationships of 1,4-bis(arylsulfonamido)-benzene or naphthalene-N,N'-diacetic acids with varying C2-substituents as inhibitors of Keap1-Nrf2 protein-protein interaction.Eur J Med Chem. 2022 Jul 5;237:114380. doi: 10.1016/j.ejmech.2022.114380. Epub 2022 Apr 13. Eur J Med Chem. 2022. PMID: 35462166 Free PMC article.
-
MAFG is a potential therapeutic target to restore chemosensitivity in cisplatin-resistant cancer cells by increasing reactive oxygen species.Transl Res. 2018 Oct;200:1-17. doi: 10.1016/j.trsl.2018.06.005. Epub 2018 Jun 30. Transl Res. 2018. PMID: 30053382 Free PMC article.
-
An internal ribosomal entry site mediates redox-sensitive translation of Nrf2.Nucleic Acids Res. 2010 Jan;38(3):778-88. doi: 10.1093/nar/gkp1048. Epub 2009 Nov 24. Nucleic Acids Res. 2010. PMID: 19934254 Free PMC article.
-
Role of Plant-Derived Compounds in the Molecular Pathways Related to Inflammation.Int J Mol Sci. 2023 Feb 28;24(5):4666. doi: 10.3390/ijms24054666. Int J Mol Sci. 2023. PMID: 36902097 Free PMC article. Review.
References
-
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. - PubMed
-
- Klaassen CD, Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309–328. - PubMed
-
- Mandlekar S, Hong JL, Kong AN. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr Drug Metab. 2006;7:661–675. - PubMed
-
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9926–9930. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

