Synaptic adhesion-like molecules (SALMs) promote neurite outgrowth
- PMID: 18585462
- PMCID: PMC2602877
- DOI: 10.1016/j.mcn.2008.05.019
Synaptic adhesion-like molecules (SALMs) promote neurite outgrowth
Abstract
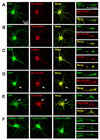
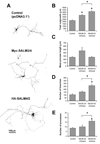
SALMs are a family of five adhesion molecules whose expression is largely restricted to the CNS. Initial reports showed that SALM1 functions in neurite outgrowth while SALM2 is involved in synapse formation. To investigate the function of SALMs in detail, we asked if all five are involved in neurite outgrowth. Expression of epitope-tagged proteins in cultured hippocampal neurons showed that SALMs are distributed throughout neurons, including axons, dendrites, and growth cones. Over-expression of each SALM resulted in enhanced neurite outgrowth, but with different phenotypes. Neurite outgrowth could be reduced by applying antibodies targeting the extracellular leucine rich regions of SALMs and with RNAi. Through over-expression of deletion constructs, we found that the C-terminal PDZ binding domains of SALMs 1-3 are required for most aspects of neurite outgrowth. In addition, by using a chimera of SALMs 2 and 4, we found that the N-terminus is also involved in neurite outgrowth.
Figures





Similar articles
-
Dileucine and PDZ-binding motifs mediate synaptic adhesion-like molecule 1 (SALM1) trafficking in hippocampal neurons.J Biol Chem. 2012 Feb 10;287(7):4470-84. doi: 10.1074/jbc.M111.279661. Epub 2011 Dec 15. J Biol Chem. 2012. PMID: 22174418 Free PMC article.
-
The SALM/Lrfn family of leucine-rich repeat-containing cell adhesion molecules.Semin Cell Dev Biol. 2011 Jul;22(5):492-8. doi: 10.1016/j.semcdb.2011.06.005. Epub 2011 Jun 29. Semin Cell Dev Biol. 2011. PMID: 21736948 Review.
-
A novel family of adhesion-like molecules that interacts with the NMDA receptor.J Neurosci. 2006 Feb 22;26(8):2174-83. doi: 10.1523/JNEUROSCI.3799-05.2006. J Neurosci. 2006. PMID: 16495444 Free PMC article.
-
Flotillin-1 mediates neurite branching induced by synaptic adhesion-like molecule 4 in hippocampal neurons.Mol Cell Neurosci. 2010 Nov;45(3):213-25. doi: 10.1016/j.mcn.2010.06.012. Epub 2010 Jun 25. Mol Cell Neurosci. 2010. PMID: 20600927
-
Synaptic organizers: synaptic adhesion-like molecules (SALMs).Curr Opin Struct Biol. 2019 Feb;54:59-67. doi: 10.1016/j.sbi.2019.01.002. Epub 2019 Feb 8. Curr Opin Struct Biol. 2019. PMID: 30743183 Review.
Cited by
-
FLRTing Neurons in Cortical Migration During Cerebral Cortex Development.Front Cell Dev Biol. 2020 Sep 17;8:578506. doi: 10.3389/fcell.2020.578506. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33043013 Free PMC article. Review.
-
Dileucine and PDZ-binding motifs mediate synaptic adhesion-like molecule 1 (SALM1) trafficking in hippocampal neurons.J Biol Chem. 2012 Feb 10;287(7):4470-84. doi: 10.1074/jbc.M111.279661. Epub 2011 Dec 15. J Biol Chem. 2012. PMID: 22174418 Free PMC article.
-
Trimester-Specific Associations of Prenatal Lead Exposure With Infant Cord Blood DNA Methylation at Birth.Epigenet Insights. 2020 Jul 20;13:2516865720938669. doi: 10.1177/2516865720938669. eCollection 2020. Epigenet Insights. 2020. PMID: 32734142 Free PMC article.
-
SALM4 suppresses excitatory synapse development by cis-inhibiting trans-synaptic SALM3-LAR adhesion.Nat Commun. 2016 Aug 2;7:12328. doi: 10.1038/ncomms12328. Nat Commun. 2016. PMID: 27480238 Free PMC article.
-
Comparative Genomic Characterization of Buffalo Fibronectin Type III Domain Proteins: Exploring the Novel Role of FNDC5/Irisin as a Ligand of Gonadal Receptors.Biology (Basel). 2021 Nov 19;10(11):1207. doi: 10.3390/biology10111207. Biology (Basel). 2021. PMID: 34827201 Free PMC article.
References
-
- Chen Y, Aulia S, Li L, Tang BL. AMIGO and friends: an emerging family of brain-enriched, neuronal growth modulating, type I transmembrane proteins with leucine-rich repeats (LRR) and cell adhesion molecule motifs. Brain Res Rev. 2006;51:265–274. - PubMed
-
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

