Role of mechanical stress in regulating airway surface hydration and mucus clearance rates
- PMID: 18585484
- PMCID: PMC2645865
- DOI: 10.1016/j.resp.2008.04.020
Role of mechanical stress in regulating airway surface hydration and mucus clearance rates
Abstract
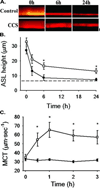
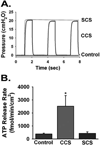
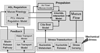
Effective clearance of mucus is a critical innate airway defense mechanism, and under appropriate conditions, can be stimulated to enhance clearance of inhaled pathogens. It has become increasingly clear that extracellular nucleotides (ATP and UTP) and nucleosides (adenosine) are important regulators of mucus clearance in the airways as a result of their ability to stimulate fluid secretion, mucus hydration, and cilia beat frequency (CBF). One ubiquitous mechanism to stimulate ATP release is through external mechanical stress. This article addresses the role of physiologically relevant mechanical forces in the lung and their effects on regulating mucociliary clearance (MCC). The effects of mechanical forces on the stimulating ATP release, fluid secretion, CBF, and MCC are discussed. Also discussed is evidence suggesting that airway hydration and stimulation of MCC by stress-mediated ATP release may play a role in several therapeutic strategies directed at improving mucus clearance in patients with obstructive lung diseases, including cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD).
Figures


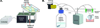

References
-
- App EM, Kieselmann R, Reinhardt D, Lindemann H, Dasgupta B, King M, Brand P. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy: flutter vs autogenic drainage. Chest. 1998;114:171–177. - PubMed
-
- Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–17138. - PubMed
-
- Basser PJ, McMahon TA, Griffith P. The mechanism of mucus clearance in cough. J Biomech Eng. 1989;111:288–297. - PubMed
-
- Bennett WD, Foster WM, Chapman WF. Cough-enhanced mucus clearance in the normal lung. J Appl Physiol. 1990;69:1670–1675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

