Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing
- PMID: 18585984
- PMCID: PMC2642887
- DOI: 10.1016/j.resp.2008.05.008
Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing
Abstract
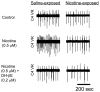
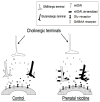
There is mounting evidence that neonatal animals exposed to nicotine in the prenatal period exhibit a variety of anatomic and functional abnormalities that adversely affect their respiratory and cardiovascular control systems, but how nicotine causes these developmental alterations is unknown. The principle that guides our work is that PNE impairs the ability of nicotinic acetylcholine receptors (nAChRs) to modulate the pre-synaptic release of both inhibitory (particularly GABA) and excitatory (glutamate) neurotransmitters, leading to marked alterations in the density and/or function of receptors on the (post-synaptic) membrane of respiratory neurons. Such changes could lead to impaired ventilatory responses to sensory afferent stimulation, and altered breathing patterns, including central apneic events. In this brief review we summarize the work that lead to the development of this hypothesis, and introduce some new data that support and extend it.
Figures

Similar articles
-
Central cholinergic regulation of respiration: nicotinic receptors.Acta Pharmacol Sin. 2009 Jun;30(6):761-70. doi: 10.1038/aps.2009.88. Acta Pharmacol Sin. 2009. PMID: 19498418 Free PMC article. Review.
-
Prenatal nicotine exposure alters medullary nicotinic and AMPA-mediated control of respiratory frequency in vitro.Respir Physiol Neurobiol. 2009 Oct 31;169(1):1-10. doi: 10.1016/j.resp.2009.07.022. Epub 2009 Aug 3. Respir Physiol Neurobiol. 2009. PMID: 19651248 Free PMC article.
-
Impacts of perinatal nicotine exposure on nicotinic acetylcholine receptor expression and glutamatergic synaptic transmission in the mouse auditory brainstem.J Physiol. 2025 May;603(9):2857-2876. doi: 10.1113/JP286971. Epub 2025 May 5. J Physiol. 2025. PMID: 40320912 Free PMC article.
-
Perinatal nicotine exposure impairs the maturation of glutamatergic inputs in the auditory brainstem.J Physiol. 2017 Jun 1;595(11):3573-3590. doi: 10.1113/JP274059. Epub 2017 Mar 10. J Physiol. 2017. PMID: 28190266 Free PMC article.
-
Nicotinic mechanisms influencing synaptic plasticity in the hippocampus.Acta Pharmacol Sin. 2009 Jun;30(6):752-60. doi: 10.1038/aps.2009.39. Epub 2009 May 11. Acta Pharmacol Sin. 2009. PMID: 19434057 Free PMC article. Review.
Cited by
-
All roads lead to inflammation: Is maternal immune activation a common culprit behind environmental factors impacting offspring neural control of breathing?Respir Physiol Neurobiol. 2020 Mar;274:103361. doi: 10.1016/j.resp.2019.103361. Epub 2019 Dec 23. Respir Physiol Neurobiol. 2020. PMID: 31874263 Free PMC article. Review.
-
Central cholinergic regulation of respiration: nicotinic receptors.Acta Pharmacol Sin. 2009 Jun;30(6):761-70. doi: 10.1038/aps.2009.88. Acta Pharmacol Sin. 2009. PMID: 19498418 Free PMC article. Review.
-
Prenatal nicotinic exposure prolongs superior laryngeal C-fiber-mediated apnea and bradycardia through enhancing neuronal TRPV1 expression and excitation.FASEB J. 2017 Oct;31(10):4325-4334. doi: 10.1096/fj.201700163R. Epub 2017 Jun 14. FASEB J. 2017. PMID: 28615326 Free PMC article.
-
Prenatal nicotine exposure alters respiratory long-term facilitation in neonatal rats.Respir Physiol Neurobiol. 2009 Dec 31;169(3):333-7. doi: 10.1016/j.resp.2009.09.015. Epub 2009 Oct 7. Respir Physiol Neurobiol. 2009. PMID: 19818419 Free PMC article.
-
Developmental nicotine exposure alters neurotransmission and excitability in hypoglossal motoneurons.J Neurophysiol. 2011 Jan;105(1):423-33. doi: 10.1152/jn.00876.2010. Epub 2010 Nov 10. J Neurophysiol. 2011. PMID: 21068261 Free PMC article.
References
-
- Bamford OS, Carroll JL. Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir Physiol. 1999;117:29–40. - PubMed
-
- Bamford OS, Schuen JN, Carroll JL. Effect of nicotine exposure on postnatal ventilatory responses to hypoxia and hypercapnia. Respir Physiol. 1996;106:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

