Purification and characterization of a novel excitatory peptide from Conus distans venom that defines a novel gene superfamily of conotoxins
- PMID: 18586046
- PMCID: PMC4492795
- DOI: 10.1016/j.toxicon.2008.05.014
Purification and characterization of a novel excitatory peptide from Conus distans venom that defines a novel gene superfamily of conotoxins
Abstract
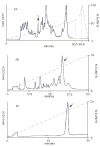
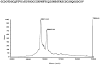
An excitatory peptide, di16a, with 49 amino acids and 10 cysteine residues was purified and characterized from the venom of Conus distans. Five AA residues were modified: one gamma-carboxyglutamate (Gla), and four hydroxyproline (Hyp) residues. A cDNA clone encoding the precursor for the peptide was characterized; the peptide has a novel cysteine framework and a distinctive signal sequence that differs from any other conotoxin superfamily. The peptide was chemically synthesized and folded, and synthetic and native materials were shown to co-elute. Injection of the synthetic peptide causes a hyperexcitable phenotype in mice greater than 3 weeks of age at lower doses, and lethargy at higher doses. The peptide defines both a previously uncharacterized gene superfamily of conopeptides, and a new Cys pattern with three vicinal Cys residues.
Figures



Similar articles
-
Definition of the M-conotoxin superfamily: characterization of novel peptides from molluscivorous Conus venoms.Biochemistry. 2005 Jun 7;44(22):8176-86. doi: 10.1021/bi047541b. Biochemistry. 2005. PMID: 15924437
-
The spasmodic peptide defines a new conotoxin superfamily.Biochemistry. 2000 Feb 22;39(7):1583-8. doi: 10.1021/bi9923712. Biochemistry. 2000. PMID: 10677206
-
Isolation and characterization of three novel Gla-containing Conus marmoreus venom peptides, one with a novel cysteine pattern.Biochem Biophys Res Commun. 2004 Jul 9;319(4):1081-7. doi: 10.1016/j.bbrc.2004.05.088. Biochem Biophys Res Commun. 2004. PMID: 15194478
-
Novel excitatory Conus peptides define a new conotoxin superfamily.J Neurochem. 2003 May;85(3):610-21. doi: 10.1046/j.1471-4159.2003.01685.x. J Neurochem. 2003. PMID: 12694387
-
Post-translationally modified conopeptides: Biological activities and pharmacological applications.Peptides. 2021 May;139:170525. doi: 10.1016/j.peptides.2021.170525. Epub 2021 Mar 5. Peptides. 2021. PMID: 33684482 Review.
Cited by
-
Apoptosis Activation in Human Lung Cancer Cell Lines by a Novel Synthetic Peptide Derived from Conus californicus Venom.Toxins (Basel). 2016 Feb 5;8(2):38. doi: 10.3390/toxins8020038. Toxins (Basel). 2016. PMID: 26861394 Free PMC article.
-
Characterization of the First Conotoxin from Conus ateralbus, a Vermivorous Cone Snail from the Cabo Verde Archipelago.Mar Drugs. 2019 Jul 24;17(8):432. doi: 10.3390/md17080432. Mar Drugs. 2019. PMID: 31344776 Free PMC article.
-
Venomics Reveals a Non-Compartmentalised Venom Gland in the Early Diverged Vermivorous Conus distans.Toxins (Basel). 2022 Mar 19;14(3):226. doi: 10.3390/toxins14030226. Toxins (Basel). 2022. PMID: 35324723 Free PMC article.
-
Optimized deep-targeted proteotranscriptomic profiling reveals unexplored Conus toxin diversity and novel cysteine frameworks.Proc Natl Acad Sci U S A. 2015 Jul 21;112(29):E3782-91. doi: 10.1073/pnas.1501334112. Epub 2015 Jul 6. Proc Natl Acad Sci U S A. 2015. PMID: 26150494 Free PMC article.
-
9.3 KDa components of the injected venom of Conus purpurascens define a new five-disulfide conotoxin framework.Biopolymers. 2011;96(2):158-65. doi: 10.1002/bip.21406. Biopolymers. 2011. PMID: 20564010 Free PMC article.
References
-
- Bandyopadhyay P. Vitamins and Hormones. Elsevier Inc; 2008. Vitamin K-Dependent gamma-Glutamylcarboxylation: an Ancient Posttranslational modification. In press. - PubMed
-
- Bandyopadhyay PK, Colledge CJ, Walker CS, Zhou LM, Hillyard DR, Olivera BM. Conantokin-G precursor and its role in γ-carboxylation by a vitamin K-dependent carboxylase from a Conus snail. J Biol Chem. 1998;273:5447–5450. - PubMed
-
- Bayrhuber M, Vijayan V, Ferber M, Graf R, Korukottu J, Imperial J, Garrett JE, Olivera BM, terlau H, Zweckstetter M, Becker S. Conkunitzin-S1 is the first member of a new Kunitz-type neurotoxin family – structural and functional characterization. J Biol Chem. 2005;180:21246–21255. - PubMed
-
- Brown MA, Begley GS, Czerwiec E, Stenberg LM, Jacobs M, Kalume DE, Roepstorff P, Stenflo J, Furie BC, Furie B. Precursors of novel Gla-containing conotoxins contain a carboxy-terminal recognition site that directs gamma-carboxylation. Biochemistry. 2005;44:9150–9159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

