Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis
- PMID: 18586680
- PMCID: PMC2527095
- DOI: 10.1074/jbc.M804116200
Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis
Abstract
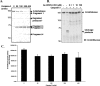
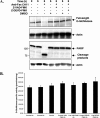
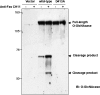
Beta-O-linked N-acetylglucosamine is a dynamic post-translational modification involved in protein regulation in a manner similar to phosphorylation. Removal of N-acetylglucosamine is regulated by beta-N-acetylglucosaminidase (O-GlcNAcase), which was previously shown to be a substrate of caspase-3 in vitro. Here we show that O-GlcNAcase is cleaved by caspase-3 into two fragments during apoptosis, an N-terminal fragment containing the O-GlcNAcase active site and a C-terminal fragment containing a region with homology to GCN5 histone acetyl-transferases. The caspase-3 cleavage site of O-GlcNAcase, mapped by Edman sequencing, is a noncanonical recognition site that occurs after Asp-413 of the SVVD sequence in human O-GlcNAcase. A point mutation, D413A, abrogates cleavage by caspase-3 both in vitro and in vivo. Finally, we show that O-GlcNAcase activity is not affected by caspase-3 cleavage because the N- and C-terminal O-GlcNAcase fragments remain associated after the cleavage. Furthermore, when co-expressed simultaneously in the same cell, the N-terminal and C-terminal caspase fragments associate to reconstitute O-GlcNAcase enzymatic activity. These studies support the identification of O-GlcNAcase as a caspase-3 substrate with a novel caspase-3 cleavage site and provide insight about O-GlcNAcase regulation during apoptosis.
Figures




References
-
- Torres, C. R., and Hart, G. W. (1984) J. Biol. Chem. 2593308 –3317 - PubMed
-
- Wells, L., Vosseller, K., and Hart, G. W. (2001) Science 2912376 –2378 - PubMed
-
- Hart, G. W., Housley, M. P., and Slawson, C. (2007) Nature 4461017 –1022 - PubMed
-
- Whelan, S. A., and Hart, G. W. (2003) Circ. Res. 931047 –1058 - PubMed
-
- Zachara, N. E., and Hart, G. W. (2002) Chem. Rev. 102431 –438 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

