Longitudinal multimodal imaging in mild to moderate Alzheimer disease: a pilot study with memantine
- PMID: 18586865
- PMCID: PMC2582338
- DOI: 10.1136/jnnp.2007.141648
Longitudinal multimodal imaging in mild to moderate Alzheimer disease: a pilot study with memantine
Abstract
Objective: To study the feasibility of multimodal neuroimaging in mild to moderate Alzheimer disease (AD) and to estimate the size of possible treatment effects of memantine on potential functional, structural and metabolic biomarkers of disease progression.
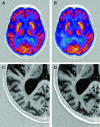
Methods: In this randomised, double-blind, placebo-controlled pilot study, 36 patients with moderate AD received 52 weeks of memantine (20 mg/day) or placebo. Patients were re-evaluated after 26 and 52 weeks to measure the change from baseline in several outcome measures including global and regional glucose metabolism, total brain and hippocampal volumes, as well as chemical shift imaging-derived global and regional N-acetylaspartate and myoinositol concentrations.
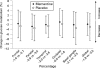
Results: In the total population, global glucose metabolism decreased by 2.3% (p<0.01), total brain volume by 2.1% (p<0.001) and hippocampal volume by 2.7% (p<0.01) after 52 weeks. Chemical shift imaging (CSI) spectra were severely affected by patient-induced artefacts and highly variable. Patients receiving memantine showed less decline in glucose metabolism in all brain areas than patients on placebo. Their loss of hippocampal volume was substantially smaller (2.4% vs 4.0%). No between-group differences were seen for changes in total brain volume.
Conclusions: The results support the use of multimodal imaging including MRI and positron emission tomography (PET) to monitor the progression of moderate AD. CSI yielded unreliable longitudinal results. The data suggest that memantine has potentially protective effects in AD and they can be used for planning larger confirmatory studies on the cerebral effects of memantine.
Conflict of interest statement
Figures
References
-
- Cummings J. Alzheimer’s disease. N Engl J Med 2004;351:56–67 - PubMed
-
- Desai A, Grossberg G. Diagnosis and treatment of Alzheimer’s disease. Neurology 2005;64:S34–9 - PubMed
-
- Schmidt R, Scheltens P, Erkinjuntti T, et al. White matter lesion progression: A surrogate endpoint for trials in cerebral small vessel disease. Neurology 2004;63:139–44 - PubMed
-
- Chen J, Charles H, Barboriak D, et al. Magnetic resonance spectroscopy in Alzheimer’s disease: focus on N-acetylaspartate. Acta Neurol Scand 2000;176Suppl:20–6 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
