Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT
- PMID: 18586884
- PMCID: PMC2519212
- DOI: 10.1152/ajpheart.01189.2007
Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT
Abstract
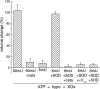
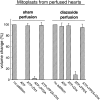
Activation of protein kinase Cepsilon (PKCepsilon), opening of mitochondrial ATP-sensitive K(+) channels (mitoK(ATP)), and increased mitochondrial reactive oxygen species (ROS) are key events in the signaling that underlies cardioprotection. We showed previously that mitoK(ATP) is opened by activation of a mitochondrial PKCepsilon, designated PKCepsilon1, that is closely associated with mitoK(ATP). mitoK(ATP) opening then causes an increase in ROS production by complex I of the respiratory chain. This ROS activates a second pool of PKCepsilon, designated PKCepsilon2, which inhibits the mitochondrial permeability transition (MPT). In the present study, we measured mitoK(ATP)-dependent changes in mitochondrial matrix volume to further investigate the relationships among PKCepsilon, mitoK(ATP), ROS, and MPT. We present evidence that 1) mitoK(ATP) can be opened by H(2)O(2) and nitric oxide (NO) and that these effects are mediated by PKCepsilon1 and not by direct actions on mitoK(ATP), 2) superoxide has no effect on mitoK(ATP) opening, 3) exogenous H(2)O(2) or NO also inhibits MPT opening, and both compounds do so independently of mitoK(ATP) activity via activation of PKCepsilon2, 4) mitoK(ATP) opening induced by PKG, phorbol ester, or diazoxide is not mediated by ROS, and 5) mitoK(ATP)-generated ROS activates PKCepsilon1 and induces phosphorylation-dependent mitoK(ATP) opening in vitro and in vivo. Thus mitoK(ATP)-dependent mitoK(ATP) opening constitutes a positive feedback loop capable of maintaining the channel open after the stimulus is no longer present. This feedback pathway may be responsible for the lasting protective effect of preconditioning, colloquially known as the memory effect.
Figures








References
-
- Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol 291: H2067–H2074, 2006. - PubMed
-
- Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Bejui F, Robert D, Ovize M. Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res 61: 115–122, 2004. - PubMed
-
- Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation 111: 194–197, 2005. - PubMed
-
- Beavis AD, Brannan RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix volume. J Biol Chem 260: 13424–13433, 1985. - PubMed
-
- Brookes PS, Salinas EP, Darley-Usmar K, Eiserich JP, Freeman BA, Darley-Usmar VM, Anderson PG. Concentration-dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome c release. J Biol Chem 275: 20474–20479, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

