Function and redundancy of the chaplin cell surface proteins in aerial hypha formation, rodlet assembly, and viability in Streptomyces coelicolor
- PMID: 18586935
- PMCID: PMC2519512
- DOI: 10.1128/JB.00685-08
Function and redundancy of the chaplin cell surface proteins in aerial hypha formation, rodlet assembly, and viability in Streptomyces coelicolor
Abstract
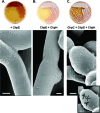
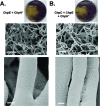
The chaplins are a family of eight secreted proteins that are critical for raising aerial hyphae in Streptomyces coelicolor. These eight chaplins can be separated into two main groups: the long chaplins (ChpA to -C) and the short chaplins (ChpD to -H). The short chaplins can be further subdivided on the basis of their abilities to form intramolecular disulfide bonds: ChpD, -F, -G, and -H contain two Cys residues, while ChpE has none. A "minimal chaplin strain" containing only chpC, chpE, and chpH was constructed and was found to raise a substantial aerial mycelium. This strain was used to examine the roles of specific chaplins. Within this strain, the Cys-containing ChpH was identified as the major polymerization unit contributing to aerial hypha formation and assembly of an intricate rodlet ultrastructure on the aerial surfaces, and the two Cys residues were determined to be critical for its function. ChpC augmented aerial hypha formation and rodlet assembly, likely by anchoring the short chaplins to the cell surface, while ChpE was essential for the viability of wild-type S. coelicolor. Interestingly, the lethal effects of a chpE null mutation could be suppressed by the loss of the other chaplins, the inactivation of the twin arginine translocation (Tat) secretion pathway, or the loss of the rodlins.
Figures





Similar articles
-
Dual amyloid domains promote differential functioning of the chaplin proteins during Streptomyces aerial morphogenesis.Proc Natl Acad Sci U S A. 2011 Jun 14;108(24):9821-6. doi: 10.1073/pnas.1018715108. Epub 2011 May 31. Proc Natl Acad Sci U S A. 2011. PMID: 21628577 Free PMC article.
-
The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor.Genes Dev. 2003 Jul 15;17(14):1727-40. doi: 10.1101/gad.264403. Epub 2003 Jun 27. Genes Dev. 2003. PMID: 12832397 Free PMC article.
-
Aerial development in Streptomyces coelicolor requires sortase activity.Mol Microbiol. 2012 Mar;83(5):992-1005. doi: 10.1111/j.1365-2958.2012.07983.x. Epub 2012 Feb 8. Mol Microbiol. 2012. PMID: 22296345
-
Regulation of Streptomyces development: reach for the sky!Trends Microbiol. 2006 Jul;14(7):313-9. doi: 10.1016/j.tim.2006.05.008. Epub 2006 Jun 6. Trends Microbiol. 2006. PMID: 16759865 Review.
-
Morphogenetic surfactants and their role in the formation of aerial hyphae in Streptomyces coelicolor.Mol Microbiol. 2006 Feb;59(3):731-42. doi: 10.1111/j.1365-2958.2005.05018.x. Mol Microbiol. 2006. PMID: 16420347 Review.
Cited by
-
A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms.Front Microbiol. 2015 Jan 13;5:745. doi: 10.3389/fmicb.2014.00745. eCollection 2014. Front Microbiol. 2015. PMID: 25628606 Free PMC article.
-
Microbial manipulation of the amyloid fold.Res Microbiol. 2012 Nov-Dec;163(9-10):592-606. doi: 10.1016/j.resmic.2012.10.009. Epub 2012 Oct 27. Res Microbiol. 2012. PMID: 23108148 Free PMC article. Review.
-
Current Understanding on Adhesion and Biofilm Development in Actinobacteria.Int J Microbiol. 2021 May 22;2021:6637438. doi: 10.1155/2021/6637438. eCollection 2021. Int J Microbiol. 2021. PMID: 34122552 Free PMC article. Review.
-
Dual amyloid domains promote differential functioning of the chaplin proteins during Streptomyces aerial morphogenesis.Proc Natl Acad Sci U S A. 2011 Jun 14;108(24):9821-6. doi: 10.1073/pnas.1018715108. Epub 2011 May 31. Proc Natl Acad Sci U S A. 2011. PMID: 21628577 Free PMC article.
-
Developmental biology of Streptomyces from the perspective of 100 actinobacterial genome sequences.FEMS Microbiol Rev. 2014 May;38(3):345-79. doi: 10.1111/1574-6976.12047. Epub 2013 Nov 19. FEMS Microbiol Rev. 2014. PMID: 24164321 Free PMC article. Review.
References
-
- Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417141-147. - PubMed
-
- Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 11643-49. - PubMed
-
- Capstick, D. S., J. M. Willey, M. J. Buttner, and M. A. Elliot. 2007. SapB and the chaplins: connections between morphogenetic proteins in Streptomyces coelicolor. Mol. Microbiol. 64602-613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

