A thioredoxin family protein of the apicoplast periphery identifies abundant candidate transport vesicles in Toxoplasma gondii
- PMID: 18586952
- PMCID: PMC2547066
- DOI: 10.1128/EC.00081-08
A thioredoxin family protein of the apicoplast periphery identifies abundant candidate transport vesicles in Toxoplasma gondii
Abstract
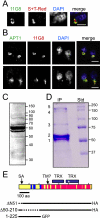
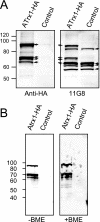
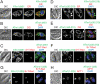
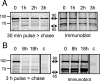
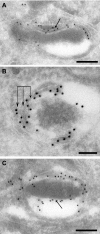
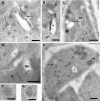
Toxoplasma gondii, which causes toxoplasmic encephalitis and birth defects, contains an essential chloroplast-related organelle to which proteins are trafficked via the secretory system. This organelle, the apicoplast, is bounded by multiple membranes. In this report we identify a novel apicoplast-associated thioredoxin family protein, ATrx1, which is predominantly soluble or peripherally associated with membranes, and which localizes primarily to the outer compartments of the organelle. As such, it represents the first protein to be identified as residing in the apicoplast intermembrane spaces. ATrx1 lacks the apicoplast targeting sequences typical of luminal proteins. However, sequences near the N terminus are required for proper targeting of ATrx1, which is proteolytically processed from a larger precursor to multiple smaller forms. This protein reveals a population of vesicles, hitherto unrecognized as being highly abundant in the cell, which may serve to transport proteins to the apicoplast.
Figures


Similar articles
-
Evolving insights into protein trafficking to the multiple compartments of the apicomplexan plastid.J Eukaryot Microbiol. 2009 May-Jun;56(3):214-20. doi: 10.1111/j.1550-7408.2009.00405.x. J Eukaryot Microbiol. 2009. PMID: 19527348 Free PMC article. Review.
-
The Dissection of SNAREs Reveals Key Factors for Vesicular Trafficking to the Endosome-like Compartment and Apicoplast via the Secretory System in Toxoplasma gondii.mBio. 2021 Aug 31;12(4):e0138021. doi: 10.1128/mBio.01380-21. Epub 2021 Aug 3. mBio. 2021. PMID: 34340555 Free PMC article.
-
Vesicles bearing Toxoplasma apicoplast membrane proteins persist following loss of the relict plastid or Golgi body disruption.PLoS One. 2014 Nov 4;9(11):e112096. doi: 10.1371/journal.pone.0112096. eCollection 2014. PLoS One. 2014. PMID: 25369183 Free PMC article.
-
Toxoplasma TgAtg8-TgAtg3 Interaction Primarily Contributes to Apicoplast Inheritance and Parasite Growth in Tachyzoite.Microbiol Spectr. 2022 Feb 23;10(1):e0149521. doi: 10.1128/spectrum.01495-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196797 Free PMC article.
-
Protein trafficking inside Toxoplasma gondii.Traffic. 2008 May;9(5):636-46. doi: 10.1111/j.1600-0854.2008.00713.x. Epub 2008 Mar 4. Traffic. 2008. PMID: 18331382 Review.
Cited by
-
The Toxoplasma monocarboxylate transporters are involved in the metabolism within the apicoplast and are linked to parasite survival.Elife. 2024 Mar 19;12:RP88866. doi: 10.7554/eLife.88866. Elife. 2024. PMID: 38502570 Free PMC article.
-
A Toxoplasma gondii mutant highlights the importance of translational regulation in the apicoplast during animal infection.Mol Microbiol. 2011 Dec;82(5):1204-16. doi: 10.1111/j.1365-2958.2011.07879.x. Epub 2011 Nov 7. Mol Microbiol. 2011. PMID: 22059956 Free PMC article.
-
Evolving insights into protein trafficking to the multiple compartments of the apicomplexan plastid.J Eukaryot Microbiol. 2009 May-Jun;56(3):214-20. doi: 10.1111/j.1550-7408.2009.00405.x. J Eukaryot Microbiol. 2009. PMID: 19527348 Free PMC article. Review.
-
Metabolic Needs and Capabilities of Toxoplasma gondii through Combined Computational and Experimental Analysis.PLoS Comput Biol. 2015 May 22;11(5):e1004261. doi: 10.1371/journal.pcbi.1004261. eCollection 2015 May. PLoS Comput Biol. 2015. PMID: 26001086 Free PMC article.
-
Oxidative Stress as a Possible Target in the Treatment of Toxoplasmosis: Perspectives and Ambiguities.Int J Mol Sci. 2021 May 27;22(11):5705. doi: 10.3390/ijms22115705. Int J Mol Sci. 2021. PMID: 34071892 Free PMC article. Review.
References
-
- Bangs, J. D., L. Uyetake, M. J. Brickman, A. E. Balber, and J. C. Boothroyd. 1993. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J. Cell Sci. 1051101-1113. - PubMed
-
- Bermudes, D., K. R. Peck, M. A. Afifi, C. J. Beckers, and K. A. Joiner. 1994. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J. Biol. Chem. 26929252-29260. - PubMed
-
- Bradley, P. J., C. Ward, S. J. Cheng, D. L. Alexander, S. Coller, G. H. Coombs, J. D. Dunn, D. J. Ferguson, S. J. Sanderson, J. M. Wastling, and J. C. Boothroyd. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 403425-34258. - PubMed
-
- Brayton, K. A., A. O. Lau, D. R. Herndon, L. Hannick, L. S. Kappmeyer, S. J. Berens, S. L. Bidwell, W. C. Brown, J. Crabtree, D. Fadrosh, T. Feldblum, H. A. Forberger, B. J. Haas, J. M. Howell, H. Khouri, H. Koo, D. J. Mann, J. Norimine, I. T. Paulsen, D. Radune, Q. Ren, R. K. Smith, Jr., C. E. Suarez, O. White, J. R. Wortman, D. P. Knowles, Jr., T. F. McElwain, and V. M. Nene. 2007. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 31401-1413. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

