Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity
- PMID: 18587008
- PMCID: PMC2537425
- DOI: 10.1096/fj.08-108274
Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity
Abstract
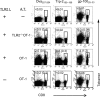
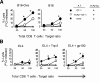
Toll-like receptors (TLRs) are among the fundamental molecules that alert the immune system to the presence of an infection by recognizing pathogen-associated molecules. Much of our understanding regarding TLR function stems from the study of innate immune cells. Recent studies by several groups, including ours, have shown that TLRs can function as costimulatory receptors for antigen-specific T cells, resulting in enhanced T-cell survival and increased expression of effector molecules. We report that the ligation of the TLR1/2 heterodimer on OT-1 cytotoxic T-lymphocytes (CTL) but not TLR2(-/-)OT-1 T cells increased cytolytic activity in vitro and in vivo. On the basis of these data, we tested the hypothesis that TLR1/2 stimulation on CTLs would enhance antitumor activity in a therapeutic model of B16-Ova melanoma. Adoptive OT-1 T-cell transfer into wild-type and MyD88(-/-) mice, followed by injection with TLR1/2 ligand, resulted in a synergistic antitumor effect, which correlated with the induction of CD8 T cells specific to various tumor antigens. In contrast, mice receiving TLR2(-/-)OT-1 T cells and TLR1/2 ligand showed minimal therapeutic efficacy. These findings emphasize the physiological significance of TLR2 engagement on CTLs and could make possible new approaches for the development of effective immunotherapies by manipulating TLR signaling within CTLs.
Figures
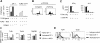

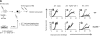
References
-
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. - PubMed
-
- Krieg A M. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. - PubMed
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. - PubMed
-
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

