Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia
- PMID: 18587396
- PMCID: PMC3525077
- DOI: 10.1038/ng.166
Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia
Abstract
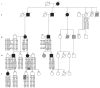
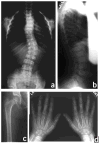
The brachyolmias constitute a clinically and genetically heterogeneous group of skeletal dysplasias characterized by a short trunk, scoliosis and mild short stature. Here, we identify a locus for an autosomal dominant form of brachyolmia on chromosome 12q24.1-12q24.2. Among the genes in the genetic interval, we selected TRPV4, which encodes a calcium permeable cation channel of the transient receptor potential (TRP) vanilloid family, as a candidate gene because of its cartilage-selective gene expression pattern. In two families with the phenotype, we identified point mutations in TRPV4 that encoded R616Q and V620I substitutions, respectively. Patch clamp studies of transfected HEK cells showed that both mutations resulted in a dramatic gain of function characterized by increased constitutive activity and elevated channel activation by either mechano-stimulation or agonist stimulation by arachidonic acid or the TRPV4-specific agonist 4alpha-phorbol 12,13-didecanoate (4alphaPDD). This study thus defines a previously unknown mechanism, activation of a calcium-permeable TRP ion channel, in skeletal dysplasia pathogenesis.
Figures
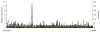


References
-
- Shohat M, Lachman R, Gruber HE, Rimoin DL. Brachyolmia: radiographic and genetic evidence of heterogeneity. Am J Med Genet. 1989;33:209–219. - PubMed
-
- Superti-Furga A, Bonafe L, Rimoin DL. Molecular-pathogenetic classification of genetic disorders of the skeleton. Am J Med Genet. 2001;106:282–293. - PubMed
-
- Horton WA, Langer LO, Collins DL, Dwyer C. Brachyolmia, recessive type (Hobaek): a clinical, radiographic, and histochemical study. Am J Med Genet. 1983;16:201–211. - PubMed
-
- Toledo SPA, et al. Recessively inherited, late onset spondylar dysplasia and peripheral corneal opacity with anomalies in urinary mucopolysaccharides: a possible error of chondroitin-6-sulfate synthesis. Am J Med Genet. 1978;2:385–395. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

