Hepatic insulin resistance directly promotes formation of cholesterol gallstones
- PMID: 18587407
- PMCID: PMC2753607
- DOI: 10.1038/nm1785
Hepatic insulin resistance directly promotes formation of cholesterol gallstones
Abstract
Despite the well-documented association between gallstones and the metabolic syndrome, the mechanistic links between these two disorders remain unknown. Here we show that mice solely with hepatic insulin resistance, created by liver-specific disruption of the insulin receptor (LIRKO mice) are markedly predisposed toward cholesterol gallstone formation due to at least two distinct mechanisms. Disinhibition of the forkhead transcription factor FoxO1, increases expression of the biliary cholesterol transporters Abcg5 and Abcg8, resulting in an increase in biliary cholesterol secretion. Hepatic insulin resistance also decreases expression of the bile acid synthetic enzymes, particularly Cyp7b1, and produces partial resistance to the farnesoid X receptor, leading to a lithogenic bile salt profile. As a result, after twelve weeks on a lithogenic diet, all of the LIRKO mice develop gallstones. Thus, hepatic insulin resistance provides a crucial link between the metabolic syndrome and increased cholesterol gallstone susceptibility.
Figures

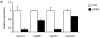
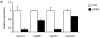




Comment in
-
Chipping away at gallstones.Nat Med. 2008 Jul;14(7):715-6. doi: 10.1038/nm0708-715. Nat Med. 2008. PMID: 18607368 No abstract available.
-
Hepatic insulin resistance ties cholesterol gallstone formation and the metabolic syndrome.Ann Hepatol. 2008 Jul-Sep;7(3):262-4. Ann Hepatol. 2008. PMID: 18753998 No abstract available.
-
Insulin and cholesterol gallstones: new insights for a complex pathogenic relationship.Hepatology. 2008 Dec;48(6):2078-80. doi: 10.1002/hep.22618. Hepatology. 2008. PMID: 19026006 No abstract available.
References
-
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Cirulation. 2002;106:3143–3421. - PubMed
-
- Diehl AK. Cholelithiasis and the insulin resistance syndrome. Hepatology. 2000;31:528–530. - PubMed
-
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. - PubMed
-
- Sandler RS, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. - PubMed
-
- Osler W. The Principles and Practice of Medicine. D. Appleton and Company; New York: 1892. p. 432.
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK073358/DK/NIDDK NIH HHS/United States
- DK45935/DK/NIDDK NIH HHS/United States
- R37 DK036588/DK/NIDDK NIH HHS/United States
- R01 DK036588/DK/NIDDK NIH HHS/United States
- L40 DK065607/DK/NIDDK NIH HHS/United States
- R01 DK073687/DK/NIDDK NIH HHS/United States
- DK063695-05/DK/NIDDK NIH HHS/United States
- DK036836-20/DK/NIDDK NIH HHS/United States
- K12 DK063696/DK/NIDDK NIH HHS/United States
- R37 DK031036/DK/NIDDK NIH HHS/United States
- DK073687/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- DK031036/DK/NIDDK NIH HHS/United States
- DK036588/DK/NIDDK NIH HHS/United States
- R01 DK045935/DK/NIDDK NIH HHS/United States
- R01 DK031036/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

