Predicting QT prolongation in humans during early drug development using hERG inhibition and an anaesthetized guinea-pig model
- PMID: 18587422
- PMCID: PMC2442905
- DOI: 10.1038/bjp.2008.267
Predicting QT prolongation in humans during early drug development using hERG inhibition and an anaesthetized guinea-pig model
Abstract
Background and purpose: Drug-induced prolongation of the QT interval can lead to torsade de pointes, a life-threatening ventricular arrhythmia. Finding appropriate assays from among the plethora of options available to predict reliably this serious adverse effect in humans remains a challenging issue for the discovery and development of drugs. The purpose of the present study was to develop and verify a reliable and relatively simple approach for assessing, during preclinical development, the propensity of drugs to prolong the QT interval in humans.
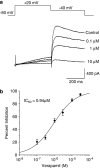
Experimental approach: Sixteen marketed drugs from various pharmacological classes with a known incidence -- or lack thereof -- of QT prolongation in humans were examined in hERG (human ether a-go-go-related gene) patch-clamp assay and an anaesthetized guinea-pig assay for QT prolongation using specific protocols. Drug concentrations in perfusates from hERG assays and plasma samples from guinea-pigs were determined using liquid chromatography-mass spectrometry.
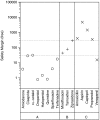
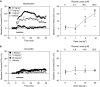
Key results: Various pharmacological agents that inhibit hERG currents prolong the QT interval in anaesthetized guinea-pigs in a manner similar to that seen in humans and at comparable drug exposures. Several compounds not associated with QT prolongation in humans failed to prolong the QT interval in this model.
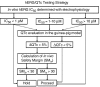
Conclusions and implications: Analysis of hERG inhibitory potency in conjunction with drug exposures and QT interval measurements in anaesthetized guinea-pigs can reliably predict, during preclinical drug development, the risk of human QT prolongation. A strategy is proposed for mitigating the risk of QT prolongation of new chemical entities during early lead optimization.
Figures

Similar articles
-
Monophasic action potential in anaesthetized guinea pigs as a biomarker for prediction of liability for drug-induced delayed ventricular repolarization.J Pharmacol Toxicol Methods. 2007 May-Jun;55(3):254-61. doi: 10.1016/j.vascn.2006.11.002. Epub 2006 Nov 28. J Pharmacol Toxicol Methods. 2007. PMID: 17229580
-
Automated electrophysiology in the preclinical evaluation of drugs for potential QT prolongation.J Pharmacol Toxicol Methods. 2005 Jul-Aug;52(1):123-35. doi: 10.1016/j.vascn.2005.04.002. J Pharmacol Toxicol Methods. 2005. PMID: 15936217
-
Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development.Cardiovasc Res. 2003 Apr 1;58(1):32-45. doi: 10.1016/s0008-6363(02)00846-5. Cardiovasc Res. 2003. PMID: 12667944 Review.
-
Early evaluation of compound QT prolongation effects: a predictive 384-well fluorescence polarization binding assay for measuring hERG blockade.J Pharmacol Toxicol Methods. 2007 May-Jun;55(3):238-47. doi: 10.1016/j.vascn.2006.09.003. Epub 2006 Oct 18. J Pharmacol Toxicol Methods. 2007. PMID: 17141530
-
Integrated risk assessment and predictive value to humans of non-clinical repolarization assays.Br J Pharmacol. 2010 Jan;159(1):115-21. doi: 10.1111/j.1476-5381.2009.00395.x. Epub 2009 Sep 25. Br J Pharmacol. 2010. PMID: 19785646 Free PMC article. Review.
Cited by
-
Age and gender dependent heart rate circadian model development and performance verification on the proarrhythmic drug case study.Theor Biol Med Model. 2013 Feb 9;10:7. doi: 10.1186/1742-4682-10-7. Theor Biol Med Model. 2013. PMID: 23394137 Free PMC article.
-
Towards Bridging Translational Gap in Cardiotoxicity Prediction: an Application of Progressive Cardiac Risk Assessment Strategy in TdP Risk Assessment of Moxifloxacin.AAPS J. 2018 Mar 14;20(3):47. doi: 10.1208/s12248-018-0199-4. AAPS J. 2018. PMID: 29541956
-
Anti-addiction drug ibogaine inhibits voltage-gated ionic currents: a study to assess the drug's cardiac ion channel profile.Toxicol Appl Pharmacol. 2013 Dec 1;273(2):259-68. doi: 10.1016/j.taap.2013.05.012. Epub 2013 May 22. Toxicol Appl Pharmacol. 2013. PMID: 23707769 Free PMC article.
-
The electro-mechanical window in anaesthetized guinea pigs: a new marker in screening for Torsade de Pointes risk.Br J Pharmacol. 2012 May;166(2):689-701. doi: 10.1111/j.1476-5381.2011.01795.x. Br J Pharmacol. 2012. PMID: 22122450 Free PMC article.
-
7-Substituted 2-Nitro-5,6-dihydroimidazo[2,1-b][1,3]oxazines: Novel Antitubercular Agents Lead to a New Preclinical Candidate for Visceral Leishmaniasis.J Med Chem. 2017 May 25;60(10):4212-4233. doi: 10.1021/acs.jmedchem.7b00034. Epub 2017 May 11. J Med Chem. 2017. PMID: 28459575 Free PMC article.
References
-
- Antzelevitch C. Tpeak−Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest. 2001;31:555–557. - PubMed
-
- Bass AS, Tomaselli G, Bullingham R, III, Kinter LB. Drugs effects on ventricular repolarization: a critical evaluation of the strengths and weaknesses of current methodologies and regulatory practices. J Pharmacol Toxicol Methods. 2005;52:12–21. - PubMed
-
- Busch AE, Malloy K, Groh WJ, Varnum MD, Adelman JP, Maylie J. The novel class III antiarrhythmics NE-10064 and NE10133 inhibit IsK channels expressed in Xenopus oocytes and IKs in guinea pig cardiac myocytes. Biochem Biophys Res Commun. 1994;202:265–270. - PubMed
-
- Carlsson L. Drug-induced torsade de pointes: the perspectives of industry. Eur Heart J (Supplement K) 2001;3:K114–K120.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

