Chordin-like 1, a bone morphogenetic protein-4 antagonist, is upregulated by hypoxia in human retinal pericytes and plays a role in regulating angiogenesis
- PMID: 18587495
- PMCID: PMC2435163
Chordin-like 1, a bone morphogenetic protein-4 antagonist, is upregulated by hypoxia in human retinal pericytes and plays a role in regulating angiogenesis
Abstract
Purpose: Pericytes play a specialized role in regulating angiogenesis and vascular function by providing vascular stability and controlling endothelial cell proliferation. Disorders in pericyte function and pericyte-endothelial interaction have been observed in several disease states including tumor angiogenesis and diabetic microangiopathy. In ischemic retinal disease, hypoxia is a potent driver of retinal angiogenesis. This study investigated the effects of hypoxia on retinal pericyte gene expression, and demonstrates a role in angiogenesis regulation for the hypoxia driven gene, chordin-like 1 (CHL-1).
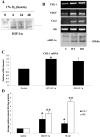
Methods: In the current studies, we investigated hypoxia-induced gene expression in human retinal pericytes and found that expression of CHL-1, a member of the bone morphogenetic protein (BMP) superfamily, is upregulated by hypoxia. We investigated regulation of CHL-1 expression and the ability of CHL-1 to antagonize the antiangiogenic properties of BMP-4 using a human cell-based angiogenesis assay.
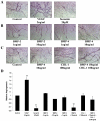
Results: We report that hypoxia induced hypoxia inducible factor-1alpha-driven expression of CHL-1. Both CHL-1 and BMP-4 were secreted from human retinal pericytes. We found that CHL-1 complexes with BMP-4 to antagonize the antiangiogenic effects of BMP-4, and that BMP-4 and vascular endothelial growth factor (VEGF) co-regulate angiogenesis.
Conclusions: We propose that hypoxia-induced upregulation of CHL-1 alters the homeostatic balance between BMP-4 and VEGF to synergize with VEGF in driving retinal angiogenesis.
Figures



Similar articles
-
Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions.Lab Invest. 1999 Apr;79(4):501-9. Lab Invest. 1999. PMID: 10212003
-
Gremlin gene expression in bovine retinal pericytes exposed to elevated glucose.Br J Ophthalmol. 2005 Dec;89(12):1638-42. doi: 10.1136/bjo.2005.069591. Br J Ophthalmol. 2005. PMID: 16299147 Free PMC article.
-
Hypoxia-induced upregulation of pigment epithelium-derived factor by retinal glial (Müller) cells.J Neurosci Res. 2012 Jan;90(1):257-66. doi: 10.1002/jnr.22732. Epub 2011 Sep 15. J Neurosci Res. 2012. PMID: 21922517
-
Pericyte-Endothelial Interactions in the Retinal Microvasculature.Int J Mol Sci. 2020 Oct 8;21(19):7413. doi: 10.3390/ijms21197413. Int J Mol Sci. 2020. PMID: 33049983 Free PMC article. Review.
-
[Aging and retinal vascular diseases].Nippon Ganka Gakkai Zasshi. 2007 Mar;111(3):207-30; discussion 231. Nippon Ganka Gakkai Zasshi. 2007. PMID: 17402563 Review. Japanese.
Cited by
-
An anteroposterior wave of vascular inhibitor downregulation signals aortae fusion along the embryonic midline axis.Development. 2010 Nov;137(21):3697-706. doi: 10.1242/dev.051664. Development. 2010. PMID: 20940228 Free PMC article.
-
CHRDL1 Regulates Stemness in Glioma Stem-like Cells.Cells. 2022 Dec 3;11(23):3917. doi: 10.3390/cells11233917. Cells. 2022. PMID: 36497175 Free PMC article.
-
Extracellular matrix proteins and tumor angiogenesis.J Oncol. 2010;2010:586905. doi: 10.1155/2010/586905. Epub 2010 Jun 29. J Oncol. 2010. PMID: 20671917 Free PMC article.
-
Site-specific genetic and functional signatures of aortic endothelial cells at aneurysm predilection sites in healthy and AngII ApoE-/- mice.Angiogenesis. 2024 Nov;27(4):719-738. doi: 10.1007/s10456-024-09933-9. Epub 2024 Jul 4. Angiogenesis. 2024. PMID: 38965173 Free PMC article.
-
Chrdl1-mediated BMP4 inhibition disrupts the balance between retinal neurons and Müller Glia.Cell Death Discov. 2024 Aug 17;10(1):367. doi: 10.1038/s41420-024-02129-6. Cell Death Discov. 2024. PMID: 39152126 Free PMC article.
References
-
- The Eye Diseases Prevalence Research Group The Prevalence of Diabetic Retinopathy Among Adults in the United States. Arch Ophthalmol. 2004;122:552–63. - PubMed
-
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–5. - PubMed
-
- Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, Ferrara N, Adamis AP. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103:1820–8. - PubMed
-
- Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
