Administration of the calcineurin inhibitor cyclosporine modulates cocaine-induced locomotor activity in rats
- PMID: 18587562
- PMCID: PMC2574760
- DOI: 10.1007/s00213-008-1189-5
Administration of the calcineurin inhibitor cyclosporine modulates cocaine-induced locomotor activity in rats
Abstract
Rationale: Cocaine administration in rats increases locomotor activity as a result of underlying changes in neurotransmitter dynamics and intracellular signaling. The serine/ threonine phosphatase, calcineurin, is known to modulate several signaling proteins that can influence behavioral responses to cocaine.
Objective: This study aimed to determine whether calcineurin plays a role in locomotor responses associated with acute and repeated cocaine exposure. Second, we examined cocaine-mediated changes in intracellular signaling to identify potential mechanism underlying the ability of calcineurin to influence cocaine-mediated behavior.
Methods: Locomotor activity was assessed over 17 days in male Sprague-Dawley rats (n = 48) that received daily administration of cocaine (15 mg/kg, s.c.) or saline in the presence or absence of the calcineurin inhibitor, cyclosporine (15 mg/kg, i.p.). Non-cocaine-treated animals from this initial experiment (n = 24) also received an acute cocaine challenge on day 18 of testing.
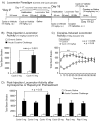
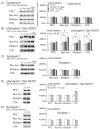
Results: Daily cyclosporine administration potentiated the locomotor response to repeated cocaine 5 min after cocaine injection and attenuated the sustained locomotor response 15 to 40 min after cocaine. Furthermore, cyclosporine pretreatment for 17 days augmented the acute locomotor response to acute cocaine 5 to 30 min after cocaine injection. Finally, repeated exposure to either cocaine or cyclosporine for 22 days increased synapsin I phosphorylation at the calcineurin-sensitive Ser 62/67 site, demonstrating a common downstream target for both calcineurin and cocaine.
Conclusion: Our results suggest that calcineurin inhibition augments locomotor responses to cocaine and mimics cocaine-mediated phosphorylation of synapsin I.
Figures

Similar articles
-
Role of calcineurin in the VTA in rats behaviorally sensitized to methamphetamine.Psychopharmacology (Berl). 2012 Mar;220(1):117-28. doi: 10.1007/s00213-011-2461-7. Epub 2011 Sep 8. Psychopharmacology (Berl). 2012. PMID: 21901318
-
Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy.Pharmacol Biochem Behav. 2005 Sep;82(1):170-81. doi: 10.1016/j.pbb.2005.08.005. Epub 2005 Sep 2. Pharmacol Biochem Behav. 2005. PMID: 16139878
-
Role of calcineurin in nicotine-mediated locomotor sensitization.J Neurosci. 2007 Aug 8;27(32):8571-80. doi: 10.1523/JNEUROSCI.2601-07.2007. J Neurosci. 2007. PMID: 17687035 Free PMC article.
-
Different locomotor sensitization responses to repeated cocaine injections are associated with differential phosphorylation of GluA1 in the dorsomedial striatum of adult rats.Behav Brain Res. 2013 Nov 15;257:71-6. doi: 10.1016/j.bbr.2013.09.038. Epub 2013 Sep 27. Behav Brain Res. 2013. PMID: 24079996
-
Cocaine-induced behavioral sensitization in the young rat.Psychopharmacology (Berl). 2000 Aug;151(2-3):291-8. doi: 10.1007/s002130000377. Psychopharmacology (Berl). 2000. PMID: 10972476
Cited by
-
Aberrant behavioral sensitization by methamphetamine in junctophilin-deficient mice.Mol Neurobiol. 2015 Apr;51(2):533-42. doi: 10.1007/s12035-014-8737-2. Epub 2014 May 22. Mol Neurobiol. 2015. PMID: 24848513
-
Treatment with the calcineurin inhibitor and immunosuppressant cyclosporine A impairs sensorimotor gating in Dark Agouti rats.Psychopharmacology (Berl). 2021 Apr;238(4):1047-1057. doi: 10.1007/s00213-020-05751-1. Epub 2020 Dec 21. Psychopharmacology (Berl). 2021. PMID: 33349900 Free PMC article.
-
Role of calcineurin in the VTA in rats behaviorally sensitized to methamphetamine.Psychopharmacology (Berl). 2012 Mar;220(1):117-28. doi: 10.1007/s00213-011-2461-7. Epub 2011 Sep 8. Psychopharmacology (Berl). 2012. PMID: 21901318
References
-
- Ahmed T, Frey JU. Plasticity-specific phosphorylation of CaMKII, MAP-kinases and CREB during late-LTP in rat hippocampal slices in vitro. Neuropharmacology. 2005;49 - PubMed
-
- Bahi A, Boyer F, Kafri T, Dreyer J. CD81-induced behavioural changes during chronic cocaine administration: in vivo gene delivery with regulatable lentivirus. Eur J Neurosci. 2004;19:1621–33. - PubMed
-
- Bahler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

