Metabolic cleavage and translocation efficiency of selected cell penetrating peptides: a comparative study with epithelial cell cultures
- PMID: 18587651
- PMCID: PMC2751379
- DOI: 10.1208/s12248-008-9029-4
Metabolic cleavage and translocation efficiency of selected cell penetrating peptides: a comparative study with epithelial cell cultures
Abstract
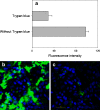
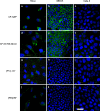
We investigated the metabolic stability of four cell penetrating peptides (CPPs), namely SAP, hCT(9-32)-br, [Palpha] and [Pbeta], when in contact with either subconfluent HeLa, confluent MDCK or Calu-3 epithelial cell cultures. Additionally, through analysis of their cellular translocation efficiency, we evaluated possible relations between metabolic stability and translocation efficiency. Metabolic degradation kinetics and resulting metabolites were assessed using RP-HPLC and MALDI-TOF mass spectrometry. Translocation efficiencies were determined using fluorescence-activated cell sorting (FACS) and confocal laser scanning microscopy (CLSM). Between HeLa, MDCK and Calu-3 we found the levels of proteolytic activities to be highly variable. However, for each peptide, the individual degradation patterns were quite similar. The metabolic stability of the investigated CPPs was in the order of CF-SAP = CF-hCT(9-32)-br > [Pbeta]-IAF > [Palpha] and we identified specific cleavage sites for each of the four peptides. Throughout, we observed higher translocation efficiencies into HeLa cells as compared to MDCK and Calu-3, corresponding to the lower state of differentiation of HeLa cell cultures. No direct relation between metabolic stability and translocation efficiency was found, indicating that metabolic stability in general is not a main limiting factor for efficient cellular translocation. Nevertheless, translocation of individual CPPs may be improved by structural modifications aiming at increased metabolic stability.
Figures



Similar articles
-
Metabolic cleavage of cell-penetrating peptides in contact with epithelial models: human calcitonin (hCT)-derived peptides, Tat(47-57) and penetratin(43-58).Biochem J. 2004 Sep 15;382(Pt 3):945-56. doi: 10.1042/BJ20040238. Biochem J. 2004. PMID: 15193145 Free PMC article.
-
Differentiation restricted endocytosis of cell penetrating peptides in MDCK cells corresponds with activities of Rho-GTPases.Pharm Res. 2007 Apr;24(4):628-42. doi: 10.1007/s11095-006-9212-1. Epub 2007 Mar 3. Pharm Res. 2007. PMID: 17334941
-
Cellular uptake but low permeation of human calcitonin-derived cell penetrating peptides and Tat(47-57) through well-differentiated epithelial models.Pharm Res. 2004 Jul;21(7):1248-56. doi: 10.1023/b:pham.0000033013.45204.c3. Pharm Res. 2004. PMID: 15290867
-
Cell-penetrating proline-rich peptidomimetics.Methods Mol Biol. 2007;386:241-67. doi: 10.1007/978-1-59745-430-8_9. Methods Mol Biol. 2007. PMID: 18604949 Review.
-
How to address CPP and AMP translocation? Methods to detect and quantify peptide internalization in vitro and in vivo (Review).Mol Membr Biol. 2007 May-Jun;24(3):173-84. doi: 10.1080/09687860601102476. Mol Membr Biol. 2007. PMID: 17520474 Review.
Cited by
-
Infection by CXCR4-Tropic Human Immunodeficiency Virus Type 1 Is Inhibited by the Cationic Cell-Penetrating Peptide Derived from HIV-1 Tat.Int J Pept. 2012;2012:349427. doi: 10.1155/2012/349427. Epub 2012 Jan 29. Int J Pept. 2012. PMID: 22319541 Free PMC article.
-
Doxorubicin in TAT peptide-modified multifunctional immunoliposomes demonstrates increased activity against both drug-sensitive and drug-resistant ovarian cancer models.Cancer Biol Ther. 2014 Jan;15(1):69-80. doi: 10.4161/cbt.26609. Epub 2013 Oct 21. Cancer Biol Ther. 2014. PMID: 24145298 Free PMC article.
-
Applications and Challenges for Use of Cell-Penetrating Peptides as Delivery Vectors for Peptide and Protein Cargos.Int J Mol Sci. 2016 Jan 30;17(2):185. doi: 10.3390/ijms17020185. Int J Mol Sci. 2016. PMID: 26840305 Free PMC article. Review.
-
Enhanced cellular uptake and metabolic stability of lipo-oligoarginine peptides.Biopolymers. 2011;96(6):772-9. doi: 10.1002/bip.21681. Epub 2011 May 31. Biopolymers. 2011. PMID: 22252426 Free PMC article.
-
Cell-penetrating TAT peptide in drug delivery systems: proteolytic stability requirements.Drug Deliv. 2011 Jul;18(5):377-84. doi: 10.3109/10717544.2011.567310. Epub 2011 Mar 26. Drug Deliv. 2011. PMID: 21438724 Free PMC article.
References
-
- Derossi D., Joliot A. H., Chassaing G., Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269:10444–10450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

