Regulation of spermatogonial stem cell self-renewal in mammals
- PMID: 18588486
- PMCID: PMC4066667
- DOI: 10.1146/annurev.cellbio.24.110707.175355
Regulation of spermatogonial stem cell self-renewal in mammals
Abstract
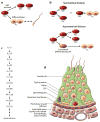
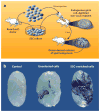
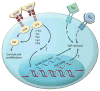
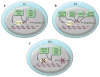
Mammalian spermatogenesis is a classic adult stem cell-dependent process, supported by self-renewal and differentiation of spermatogonial stem cells (SSCs). Studying SSCs provides a model to better understand adult stem cell biology, and deciphering the mechanisms that control SSC functions may lead to treatment of male infertility and an understanding of the etiology of testicular germ cell tumor formation. Self-renewal of rodent SSCs is greatly influenced by the niche factor glial cell line-derived neurotrophic factor (GDNF). In mouse SSCs, GDNF activation upregulates expression of the transcription factor-encoding genes bcl6b, etv5, and lhx1, which influence SSC self-renewal. Additionally, the non-GDNF-stimulated transcription factors Plzf and Taf4b have been implicated in regulating SSC functions. Together, these molecules are part of a robust gene network controlling SSC fate decisions that may parallel the regulatory networks in other adult stem cell populations.
Figures
References
-
- Airaksinen MS, Saarma M. The GDNF family: signaling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. - PubMed
-
- Aponte PM, Soda T, van de Kant HJ, de Rooij DG. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology. 2005;65:1828–47. - PubMed
-
- Barnes D, Sato G. Serum-free cell culture: a unifying approach. Cell. 1980;22:649–55. - PubMed
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

