Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain
- PMID: 18588860
- PMCID: PMC2601566
- DOI: 10.1016/j.brainres.2008.05.086
Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain
Abstract
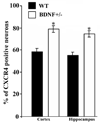
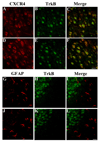
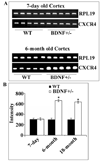
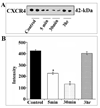
Chemokine receptors, and in particular CXCR4 and CCR5 play a key role in the neuropathogenesis of Human Immunodeficiency Virus-1 (HIV)4 associated dementia (HAD). Thus, new insight into the expression of CXCR4 in the central nervous system may help develop therapeutic compounds against HAD. Brain-derived neurotrophic factor (BDNF) is neuroprotective in vitro against two strains of the HIV envelope protein gp120 that binds to CXCR4 or CCR5. Therefore, we examined whether BDNF modulates chemokine receptor expression in vivo. The content of CXCR4 mRNA and proteins was determined in the cerebral cortex and hippocampus of 6-month-old BDNF heterozygous mice and wild type littermates by using polymerase chain reaction and immunohistochemistry, respectively. BDNF heterozygous mice exhibited an increase in CXCR4 mRNA compared to wild type. Histological analyses revealed an up-regulation of CXCR4 immunoreactivity mainly in neurons. Most of these neurons were positive for TrkB, the BDNF receptor with a tyrosine kinase activity. Increases in CXCR4 mRNA levels were observed in 18-month-old BDNF heterozygous mice but not in 7-day-old mice, suggesting that the modulatory role of BDNF occurs only in mature animals. To determine whether BDNF affects also CXCR4 internalization, SH-SY5Y neuroblastoma cells were exposed to BDNF and cell surface CXCR4 levels were measured at various times. BDNF induced CXCR4 internalization within minutes. Lastly, BDNF heterozygous mice showed higher levels of CCR5 and CXCR3 mRNA than wild type in the cerebral cortex, hippocampus and striatum. Our data indicate that BDNF may modulate the availability of chemokine receptors implicated in HIV infection.
Figures




References
-
- Bachis A, Mocchetti I. Brain-derived neurotrophic factor is neuroprotective against human immunodeficiency virus-1 envelope proteins. Ann NY Acad Sci. 2005;1053:247–257. - PubMed
-
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. - PubMed
-
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Parsadaniantz SM. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–1671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

