Similarities and differences between frozen-hydrated, rigor acto-S1 complexes of insect flight and chicken skeletal muscles
- PMID: 18588896
- PMCID: PMC2567842
- DOI: 10.1016/j.jmb.2008.06.029
Similarities and differences between frozen-hydrated, rigor acto-S1 complexes of insect flight and chicken skeletal muscles
Abstract
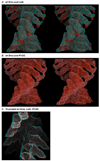
The structure and function of myosin crossbridges in asynchronous insect flight muscle (IFM) have been elucidated in situ using multiple approaches. These include generating "atomic" models of myosin in multiple contractile states by rebuilding the crystal structure of chicken subfragment 1 (S1) to fit IFM crossbridges in lower-resolution electron microscopy tomograms and by "mapping" the functional effects of genetically substituted, isoform-specific domains, including the converter domain, in chimeric IFM myosin to sequences in the crystal structure of chicken S1. We prepared helical reconstructions (approximately 25 A resolution) to compare the structural characteristics of nucleotide-free myosin0 S1 bound to actin (acto-S1) isolated from chicken skeletal muscle (CSk) and the flight muscles of Lethocerus (Leth) wild-type Drosophila (wt Dros) and a Drosophila chimera (IFI-EC) wherein the converter domain of the indirect flight muscle myosin isoform has been replaced by the embryonic skeletal myosin converter domain. Superimposition of the maps of the frozen-hydrated acto-S1 complexes shows that differences between CSk and IFM S1 are limited to the azimuthal curvature of the lever arm: the regulatory light-chain (RLC) region of chicken skeletal S1 bends clockwise (as seen from the pointed end of actin) while those of IFM S1 project in a straight radial direction. All the IFM S1s are essentially identical other than some variation in the azimuthal spread of density in the RLC region. This spread is most pronounced in the IFI-EC S1, consistent with proposals that the embryonic converter domain increases the compliance of the IFM lever arm affecting the function of the myosin motor. These are the first unconstrained models of IFM S1 bound to actin and the first direct comparison of the vertebrate and invertebrate skeletal myosin II classes, the latter for which, data on the structure of discrete acto-S1 complexes, are not readily available.
Figures



Similar articles
-
Flexibility within the heads of muscle myosin-2 molecules.J Mol Biol. 2014 Feb 20;426(4):894-907. doi: 10.1016/j.jmb.2013.11.028. Epub 2013 Dec 9. J Mol Biol. 2014. PMID: 24333017 Free PMC article.
-
Electron tomography of insect flight muscle in rigor and AMPPNP at 23 degrees C.J Mol Biol. 1996 Nov 29;264(2):279-301. doi: 10.1006/jmbi.1996.0641. J Mol Biol. 1996. PMID: 8951377
-
Visualizing myosin's power stroke in muscle contraction.J Cell Sci. 2000 Oct;113 ( Pt 20):3551-62. doi: 10.1242/jcs.113.20.3551. J Cell Sci. 2000. PMID: 11017871 Review.
-
Conformation of myosin interdomain interactions during contraction: deductions from muscle fibers using polarized fluorescence.Biochemistry. 2001 Apr 17;40(15):4821-33. doi: 10.1021/bi002387o. Biochemistry. 2001. PMID: 11294650
-
Myosin isoforms show unique conformations in the actin-bound state.Proc Natl Acad Sci U S A. 2003 Mar 18;100(6):3227-32. doi: 10.1073/pnas.0536510100. Epub 2003 Feb 28. Proc Natl Acad Sci U S A. 2003. PMID: 12612343 Free PMC article.
Cited by
-
Electron tomography of cryofixed, isometrically contracting insect flight muscle reveals novel actin-myosin interactions.PLoS One. 2010 Sep 9;5(9):e12643. doi: 10.1371/journal.pone.0012643. PLoS One. 2010. PMID: 20844746 Free PMC article.
-
Transgenic expression and purification of myosin isoforms using the Drosophila melanogaster indirect flight muscle system.Methods. 2012 Jan;56(1):25-32. doi: 10.1016/j.ymeth.2011.12.002. Epub 2011 Dec 8. Methods. 2012. PMID: 22178692 Free PMC article.
-
Structural changes in isometrically contracting insect flight muscle trapped following a mechanical perturbation.PLoS One. 2012;7(6):e39422. doi: 10.1371/journal.pone.0039422. Epub 2012 Jun 25. PLoS One. 2012. PMID: 22761792 Free PMC article.
-
Myosin S2 origins track evolution of strong binding on actin by azimuthal rolling of motor domain.Biophys J. 2015 Mar 24;108(6):1495-1502. doi: 10.1016/j.bpj.2014.12.059. Biophys J. 2015. PMID: 25809262 Free PMC article.
-
Flexibility within the heads of muscle myosin-2 molecules.J Mol Biol. 2014 Feb 20;426(4):894-907. doi: 10.1016/j.jmb.2013.11.028. Epub 2013 Dec 9. J Mol Biol. 2014. PMID: 24333017 Free PMC article.
References
-
- Squire JM, Al-Khayat HA, Harford JJ, Hudson L, Irving T, Knupp C, Reedy MK. Modelling muscle motor conformations using low-angle X-ray diffraction. IEE Proc Nanobiotechnol. 2003;150:103–110. - PubMed
-
- Taylor KA, Schmitz H, Reedy MC, Goldman YE, Franzini-Armstrong C, Sasaki H, Tregear RT, Poole K, Lucaveche C, Edwards RJ, Chen LF, Winkler H, Reedy MK. Tomographic 3D reconstruction of quick-frozen, Ca2+-activated contracting insect flight muscle. Cell. 1999;99:421–431. - PubMed
-
- Liu J, Reedy MC, Goldman YE, Franzini-Armstrong C, Sasaki H, Tregear RT, Lucaveche C, Winkler H, Baumann BA, Squire JM, Irving TC, Reedy MK, Taylor KA. Electron tomography of fast frozen, stretched rigor fibers reveals elastic distortions in the myosin crossbridges. J Struct Biol. 2004;147:268–282. - PubMed
-
- Reedy MC. Visualizing myosin's power stroke in muscle contraction. J Cell Sci. 2000;113(Pt 20):3551–3562. - PubMed
-
- Squire JM, Knupp C, Roessle M, Al-Khayat HA, Irving TC, Eakins F, Mok NS, Harford JJ, Reedy MK. X-ray diffraction studies of striated muscles. Adv Exp Med Biol. 2005;565:45–60. discussion 359–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR039155/AR/NIAMS NIH HHS/United States
- R37 GM032443/GM/NIGMS NIH HHS/United States
- P41 RR017573/RR/NCRR NIH HHS/United States
- P41 RR-01081/RR/NCRR NIH HHS/United States
- R01 GM032443/GM/NIGMS NIH HHS/United States
- P41 RR001081/RR/NCRR NIH HHS/United States
- R37 AR-14317/AR/NIAMS NIH HHS/United States
- P41 RR-17573/RR/NCRR NIH HHS/United States
- R25 GM058906/GM/NIGMS NIH HHS/United States
- GM-32443/GM/NIGMS NIH HHS/United States
- P50 GM066050/GM/NIGMS NIH HHS/United States
- AR-39155/AR/NIAMS NIH HHS/United States
- R37 AR014317/AR/NIAMS NIH HHS/United States
- R01 AR014317/AR/NIAMS NIH HHS/United States
- 5P50 GM066050/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

