How calmodulin interacts with the adenosine A(2A) and the dopamine D(2) receptors
- PMID: 18590318
- PMCID: PMC2538563
- DOI: 10.1021/pr8001782
How calmodulin interacts with the adenosine A(2A) and the dopamine D(2) receptors
Abstract
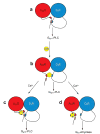
Receptor heteromerization is a mechanism used by G protein-coupled receptors to diversify their properties and function. We previously demonstrated that these interactions occur through salt bridge formation between epitopes of the involved receptors. Recent studies claim that calmodulin (CaM) binds to an Arg-rich epitope located in the amino-terminus of the dopamine D(2) receptor third intracellular loop. This is the same epitope involved in adenosine A(2A)-D(2) receptor heteromerization, through Coulombic interaction between the Arg residues and a phosphorylated serine (pS) located in the medial segment of the C-terminus of the A(2A) receptor. Mass spectrometric analysis indicates that an electrostatic interaction involving the D(2) receptor Arg-rich epitope and several CaM acidic epitopes are mainly responsible for the D(2) receptor-CaM binding. CaM could also form multiple noncovalent complexes by means of electrostatic interactions with an epitope localized in the proximal segment of the C-terminus of the A(2A) receptor. Ca(2+) disrupted the binding of CaM to the D(2) but not to the A(2A) receptor epitope, and CaM disrupted the electrostatic interactions between the D(2) receptor epitope and the more distal A(2A) receptor epitope. A model is introduced with the possible functional implications of A(2A)-D(2)-CaM interactions. These in vitro findings imply a possible regulatory role for CaM in receptor heteromers formation.
Figures




References
-
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Pharmacol Rev. 2003;55:509–550. - PubMed
-
- Ferré S, Ciruela F, Woods AS, Lluis C, Franco R. Trends Neurosci. 2007;30:440–446. - PubMed
-
- Ciruela F, Burgueno J, Casado V, Canals M, Marcelino D, Goldberg SR, Bader M, Fuxe K, Agnati LF, Lluis C, Franco R, Ferré S, Woods AS. Anal Chem. 2004;76:5354–5363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

