The effect of surface demineralization of cortical bone allograft on the properties of recombinant adeno-associated virus coatings
- PMID: 18590929
- PMCID: PMC2570090
- DOI: 10.1016/j.biomaterials.2008.06.007
The effect of surface demineralization of cortical bone allograft on the properties of recombinant adeno-associated virus coatings
Abstract
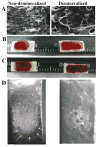
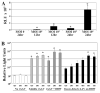
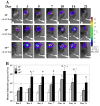
Freeze-dried recombinant adeno-associated virus (rAAV) coated structural allografts have emerged as an approach to engender necrotic cortical bone with host factors that will persist for weeks following surgery to facilitate revascularization, osteointegration, and remodeling. However, one major limitation is the nonporous cortical surface that prohibits uniform distribution of the rAAV coating prior to freeze-drying. To overcome this we have developed a demineralization method to increase surface absorbance while retaining the structural integrity of the allograft. Demineralized bone wafers (DBW) made from human femoral allograft rings demonstrated a significant 21.1% (73.6+/-3.9% versus 52.5+/-2.6%; p<0.001) increase in percent surface area coating versus mineralized controls. Co-incubation of rAAV-luciferase (rAAV-Luc) coated DBW with a monolayer of C3H10T1/2 cells in culture led to peak luciferase levels that were not significantly different from soluble rAAV-Luc controls (p>0.05), although the peaks occurred at 60h and 12h, respectively. To assess the transduction efficiency of rAAV-Luc coated DBW in vivo, we first performed a dose response with allografts containing 10(7), 10(9) or 10(10) particles that were surgically implanted into the quadriceps of mice, and assayed by in vivo bioluminescence imaging (BLI) on days 1, 3, 5, 7, 10, 14, and 21. The results demonstrated a dose response in which the DBW coated with 10(10) rAAV-Luc particles achieved peak gene expression levels on day 3, which persisted until day 21, and was significantly greater than the 10(7) dose throughout this time period (p<0.01). A direct comparison of mineralized versus DBW coated with 10(10) rAAV-Luc particles failed to demonstrate any significant differences in transduction kinetics or efficiency in vivo. Thus, surface demineralization of human cortical bone allograft increases its absorbance for uniform rAAV coating, without affecting vector transduction efficiency.
Figures

Similar articles
-
Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur.Mol Ther. 2011 Aug;19(8):1416-25. doi: 10.1038/mt.2010.294. Epub 2011 Jan 4. Mol Ther. 2011. PMID: 21206485 Free PMC article.
-
Biological effects of rAAV-caAlk2 coating on structural allograft healing.Mol Ther. 2005 Aug;12(2):212-8. doi: 10.1016/j.ymthe.2005.02.026. Mol Ther. 2005. PMID: 16043092
-
Adeno-associated virus-coated allografts: a novel approach for cranioplasty.J Tissue Eng Regen Med. 2012 Nov;6(10):e43-50. doi: 10.1002/term.1594. Epub 2012 Sep 3. J Tissue Eng Regen Med. 2012. PMID: 22941779 Free PMC article.
-
Recent advances in gene delivery for structural bone allografts.Tissue Eng. 2007 Aug;13(8):1973-85. doi: 10.1089/ten.2006.0107. Tissue Eng. 2007. PMID: 17518728 Review.
-
Gene therapy methods in bone and joint disorders. Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing.Acta Orthop Suppl. 2007 Apr;78(325):1-64. Acta Orthop Suppl. 2007. PMID: 17427340 Review.
Cited by
-
Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair.Cell Tissue Res. 2012 Mar;347(3):575-88. doi: 10.1007/s00441-011-1197-3. Epub 2011 Jun 22. Cell Tissue Res. 2012. PMID: 21695398 Free PMC article.
-
Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur.Mol Ther. 2011 Aug;19(8):1416-25. doi: 10.1038/mt.2010.294. Epub 2011 Jan 4. Mol Ther. 2011. PMID: 21206485 Free PMC article.
-
The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720.Biomaterials. 2010 Sep;31(25):6417-24. doi: 10.1016/j.biomaterials.2010.04.061. Biomaterials. 2010. PMID: 20621764 Free PMC article.
-
Musculoskeletal molecular imaging: a comprehensive overview.Trends Biotechnol. 2010 Feb;28(2):93-101. doi: 10.1016/j.tibtech.2009.11.004. Epub 2010 Jan 4. Trends Biotechnol. 2010. PMID: 20045210 Free PMC article. Review.
-
Recent tissue engineering-based advances for effective rAAV-mediated gene transfer in the musculoskeletal system.Bioengineered. 2016 Apr;7(3):175-88. doi: 10.1080/21655979.2016.1187347. Bioengineered. 2016. PMID: 27221233 Free PMC article. Review.
References
-
- Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70:369–376. - PubMed
-
- Berrey BH, Jr, Lord CF, Gebhardt MC, Mankin HJ. Fractures of allografts. Frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;72:825–833. - PubMed
-
- Enneking WF, Campanacci DA. Retrieved human allografts : a clinicopathological study. J Bone Joint Surg Am. 2001;83-A:971–986. - PubMed
-
- Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, et al. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98. - PubMed
-
- Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK 065988/DK/NIDDK NIH HHS/United States
- EY 005951/EY/NEI NIH HHS/United States
- R01 AR051469/AR/NIAMS NIH HHS/United States
- R21 DE017096/DE/NIDCR NIH HHS/United States
- R01 EY005951/EY/NEI NIH HHS/United States
- R01 DE019902/DE/NIDCR NIH HHS/United States
- AR 54041/AR/NIAMS NIH HHS/United States
- R01 AR067859/AR/NIAMS NIH HHS/United States
- AR 46545/AR/NIAMS NIH HHS/United States
- P30 DK065988/DK/NIDDK NIH HHS/United States
- P01 HL051818/HL/NHLBI NIH HHS/United States
- R21 DE026256/DE/NIDCR NIH HHS/United States
- R01 AR046545/AR/NIAMS NIH HHS/United States
- P50 AR054041/AR/NIAMS NIH HHS/United States
- AR 51469/AR/NIAMS NIH HHS/United States
- DE 17096/DE/NIDCR NIH HHS/United States
- HL 51818/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

