Neuroimaging of mitochondrial disease
- PMID: 18590986
- PMCID: PMC2600593
- DOI: 10.1016/j.mito.2008.05.003
Neuroimaging of mitochondrial disease
Abstract
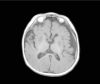
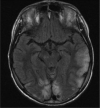
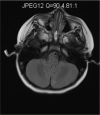
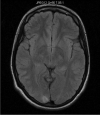
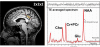
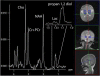
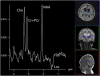
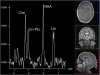
Mitochondrial disease represents a heterogeneous group of genetic disorders that require a variety of diagnostic tests for proper determination. Neuroimaging may play a significant role in diagnosis. The various modalities of nuclear magnetic resonance imaging (MRI) allow for multiple independent detection procedures that can give important anatomical and metabolic clues for diagnosis. The non-invasive nature of neuroimaging also allows for longitudinal studies. To date, no pathonmonic correlation between specific genetic defect and neuroimaging findings have been described. However, certain neuroimaging results can give important clues that a patient may have a mitochondrial disease. Conventional MRI may show deep gray structural abnormalities or stroke-like lesions that do not respect vascular territories. Chemical techniques such as proton magnetic resonance spectroscopy (MRS) may demonstrate high levels of lactate or succinate. When found, these results are suggestive of a mitochondrial disease. MRI and MRS studies may also show non-specific findings such as delayed myelination or non-specific leukodystrophy picture. However, in the context of other biochemical, structural, and clinical findings, even non-specific findings may support further diagnostic testing for potential mitochondrial disease. Once a diagnosis has been established, these non-invasive tools can also aid in following disease progression and evaluate the effects of therapeutic interventions.
Figures










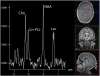
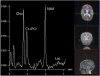

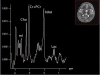

References
-
- Abe K. Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology. 2004;53:2458. - PubMed
-
- Abu-Amero KK, Bosley TM, Bohlega S, Hansen E. Mitochondrial T9957C mutation in association with NAION and seizures but not MELAS. Ophthalmic Genet. 2005;26:31–36. - PubMed
-
- Ackrell BAC, Johnson MK, Gunsalus RP, Cecchini G. Structure and function of succinate dehydrogenase and fumarate reductase. In: Muller F, editor. Biochemistry of flavoenzymes. CRC; Boca Raton, FL: 1992. pp. 229–297.
-
- Apostolova LG, White M, Moore SA, Davis PH. Deep white matter pathologic features in watershed regions: a novel pattern of central nervous system involvement in MELAS. Arch. Neurol. 2005;62:1154–1156. - PubMed
-
- Barbiroli B, Frassineti C, Martinelli P, Lotti S, Lodi R, Cortelli P, Montagna P. Coenzyme Q10 improves mitochondrial respiration in patients with mitochondrial cytopathies. An in vivo study on brain and skeletal muscle by phosphorous magnetic resonance spectroscopy. Cell Mol. Biol. (Noisy-le-grand) 1997;43:741–749. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

