Regulation of quantal shape by Rab3A: evidence for a fusion pore-dependent mechanism
- PMID: 18591190
- PMCID: PMC2538918
- DOI: 10.1113/jphysiol.2008.151191
Regulation of quantal shape by Rab3A: evidence for a fusion pore-dependent mechanism
Abstract
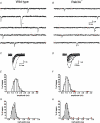
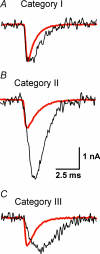
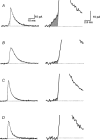
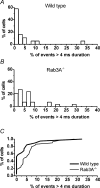
The function of Rab3A, a small GTPase located on synaptic vesicles, is not well understood. Studies in the Rab3A(-/-) mouse support a role in activity-dependent plasticity, but have not reported any effects on spontaneously occurring miniature synaptic currents, except that there is a decrease in resting frequency at the neuromuscular junction. Therefore we were surprised to find an increase in the occurrence of mEPCs with abnormally long half-widths at the neuromuscular junctions of Rab3A(-/-) mice. The abnormal miniature endplate currents (mEPCs), which have significantly greater charge than the average mEPCs for the same fibres, could arise from larger vesicles. However, the type of mEPC most increased in Rab3A(-/-) mice has a slow rise, which suggests it is not the result of full collapse fusion. To test if the slow mEPCs increased after loss of Rab3A could be due to malfunctioning fusion pores, we used carbon fibre amperometry to record pre-spike feet, which have been shown to correspond to the initial opening of a narrow fusion pore, in adrenal chromaffin cells of wild-type and Rab3A(-/-) mice. We found that small amplitude pre-spike feet with abnormally long durations were increased in Rab3A(-/-) cells. The correspondence between mEPC and amperometric data supports our interpretation that slow rising, long half-width mEPCs are caused by reduced diameter fusion pores that remain open longer. These data could be explained by a direct action of Rab3A on the fusion pore, or by Rab3A-dependent control of vesicles with unusual fusion pore characteristics.
Figures






Similar articles
-
Impaired activity-dependent plasticity of quantal amplitude at the neuromuscular junction of Rab3A deletion and Rab3A earlybird mutant mice.J Neurosci. 2011 Mar 9;31(10):3580-8. doi: 10.1523/JNEUROSCI.5278-10.2011. J Neurosci. 2011. PMID: 21389214 Free PMC article.
-
Rab3a-mediated vesicle recruitment regulates short-term plasticity at the mouse diaphragm synapse.Mol Cell Neurosci. 2009 Jun;41(2):286-96. doi: 10.1016/j.mcn.2009.03.008. Epub 2009 Apr 5. Mol Cell Neurosci. 2009. PMID: 19348946
-
Rab3a deletion reduces vesicle docking and transmitter release at the mouse diaphragm synapse.Neuroscience. 2007 Aug 10;148(1):1-6. doi: 10.1016/j.neuroscience.2007.06.011. Epub 2007 Jul 20. Neuroscience. 2007. PMID: 17640821
-
The unitary evoked potential at the frog nerve-muscle junction results from synchronous gating of fusion pores at docked vesicles.Cell Biol Int. 1999;23(8):527-32. doi: 10.1006/cbir.1999.0411. Cell Biol Int. 1999. PMID: 10704236 Review.
-
Mechanisms of synaptic vesicle exocytosis.Annu Rev Cell Dev Biol. 2000;16:19-49. doi: 10.1146/annurev.cellbio.16.1.19. Annu Rev Cell Dev Biol. 2000. PMID: 11031229 Review.
Cited by
-
Homeostatic Plasticity of the Mammalian Neuromuscular Junction.Adv Neurobiol. 2022;28:111-130. doi: 10.1007/978-3-031-07167-6_5. Adv Neurobiol. 2022. PMID: 36066823
-
Impaired activity-dependent plasticity of quantal amplitude at the neuromuscular junction of Rab3A deletion and Rab3A earlybird mutant mice.J Neurosci. 2011 Mar 9;31(10):3580-8. doi: 10.1523/JNEUROSCI.5278-10.2011. J Neurosci. 2011. PMID: 21389214 Free PMC article.
-
Homeostatic Synaptic Plasticity of Miniature Excitatory Postsynaptic Currents in Mouse Cortical Cultures Requires Neuronal Rab3A.bioRxiv [Preprint]. 2025 Mar 21:2023.06.14.544980. doi: 10.1101/2023.06.14.544980. bioRxiv. 2025. PMID: 39071374 Free PMC article. Preprint.
-
Homeostatic synaptic plasticity at the neuromuscular junction in myasthenia gravis.Ann N Y Acad Sci. 2018 Jan;1412(1):170-177. doi: 10.1111/nyas.13472. Epub 2017 Oct 5. Ann N Y Acad Sci. 2018. PMID: 28981978 Free PMC article. Review.
-
The Rab3A-22A Chimera Prevents Sperm Exocytosis by Stabilizing Open Fusion Pores.J Biol Chem. 2016 Oct 28;291(44):23101-23111. doi: 10.1074/jbc.M116.729954. Epub 2016 Sep 9. J Biol Chem. 2016. PMID: 27613869 Free PMC article.
References
-
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
-
- Ales E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. - PubMed
-
- Ardiles AO, Gonzalez-Jamett AM, Maripillan J, Naranjo D, Caviedes P, Cardenas AM. Calcium channel subtypes differentially regulate fusion pore stability and expansion. J Neurochem. 2007;103:1574–1581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

