Stages of meningococcal sepsis simulated in vitro, with emphasis on complement and Toll-like receptor activation
- PMID: 18591229
- PMCID: PMC2519403
- DOI: 10.1128/IAI.00195-08
Stages of meningococcal sepsis simulated in vitro, with emphasis on complement and Toll-like receptor activation
Abstract
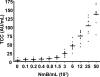
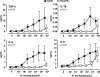
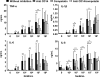
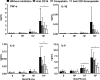
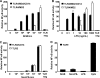
The clinical presentation of meningococcal disease is closely related to the number of meningococci in the circulation. This study aimed to examine the activation of the innate immune system after being exposed to increasing and clinically relevant concentrations of meningococci. We incubated representative Neisseria meningitidis serogroup B (ST-32) and serogroup C (ST-11) strains and a lipopolysaccharide (LPS)-deficient mutant (the 44/76 lpxA mutant) in human serum and whole blood and measured complement activation and cytokine secretion and the effect of blocking these systems. HEK293 cells transfected with Toll-like receptors (TLRs) were examined for activation of NF-kappaB. The threshold for cytokine secretion and activation of NF-kappaB was 10(3) to 10(4) meningococci/ml. LPS was the sole inflammation-inducing molecule at concentrations up to 10(5) to 10(6) meningococci/ml. The activation was dependent on TLR4-MD2-CD14. Complement contributed to the inflammatory response at >or=10(5) to 10(6) meningococci/ml, and complement activation increased exponentially at >or=10(7) bacteria/ml. Non-LPS components initiated TLR2-mediated activation at >or=10(7) bacteria/ml. As the bacterial concentration exceeded 10(7)/ml, TLR4 and TLR2 were increasingly activated, independent of CD14. In this model mimicking human disease, the inflammatory response to N. meningitidis was closely associated with the bacterial concentration. Therapeutically, CD14 inhibition alone was most efficient at a low bacterial concentration, whereas addition of a complement inhibitor may be beneficial when the bacterial load increases.
Figures
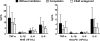
Similar articles
-
Neisseria meningitidis can induce pro-inflammatory cytokine production via pathways independent from CD14 and toll-like receptor 4.Eur Cytokine Netw. 2002 Oct-Dec;13(4):411-7. Eur Cytokine Netw. 2002. PMID: 12517725
-
Complement activation and complement-dependent inflammation by Neisseria meningitidis are independent of lipopolysaccharide.Infect Immun. 2004 Jun;72(6):3344-9. doi: 10.1128/IAI.72.6.3344-3349.2004. Infect Immun. 2004. PMID: 15155639 Free PMC article.
-
A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR4/MD2.J Infect Dis. 2001 Jan 1;183(1):89-96. doi: 10.1086/317647. Epub 2000 Nov 10. J Infect Dis. 2001. PMID: 11076707
-
Host response to Neisseria meningitidis lacking lipopolysaccharides.Expert Rev Anti Infect Ther. 2003 Dec;1(4):589-96. doi: 10.1586/14787210.1.4.589. Expert Rev Anti Infect Ther. 2003. PMID: 15482156 Review.
-
Hypothesis: combined inhibition of complement and CD14 as treatment regimen to attenuate the inflammatory response.Adv Exp Med Biol. 2008;632:253-63. Adv Exp Med Biol. 2008. PMID: 19025127 Review.
Cited by
-
The key roles of complement and tissue factor in Escherichia coli-induced coagulation in human whole blood.Clin Exp Immunol. 2015 Oct;182(1):81-9. doi: 10.1111/cei.12663. Epub 2015 Aug 2. Clin Exp Immunol. 2015. PMID: 26241501 Free PMC article.
-
Anticoagulants impact on innate immune responses and bacterial survival in whole blood models of Neisseria meningitidis infection.Sci Rep. 2018 Jul 5;8(1):10225. doi: 10.1038/s41598-018-28583-8. Sci Rep. 2018. PMID: 29977064 Free PMC article.
-
Combined inhibition of C5 and CD14 efficiently attenuated the inflammatory response in a porcine model of meningococcal sepsis.J Intensive Care. 2017 Feb 27;5:21. doi: 10.1186/s40560-017-0217-0. eCollection 2017. J Intensive Care. 2017. PMID: 28261486 Free PMC article.
-
Whole-blood incubation with the Neisseria meningitidis lpxL1 mutant induces less pro-inflammatory cytokines than the wild type, and IL-10 reduces the MyD88-dependent cytokines.Innate Immun. 2018 Feb;24(2):101-111. doi: 10.1177/1753425917749299. Epub 2018 Jan 9. Innate Immun. 2018. PMID: 29313733 Free PMC article.
-
Meningococcal disease and the complement system.Virulence. 2014 Jan 1;5(1):98-126. doi: 10.4161/viru.26515. Epub 2013 Oct 8. Virulence. 2014. PMID: 24104403 Free PMC article. Review.
References
-
- Akashi, S., S. Saitoh, Y. Wakabayashi, T. Kikuchi, N. Takamura, Y. Nagai, Y. Kusumoto, K. Fukase, S. Kusumoto, Y. Adachi, A. Kosugi, and K. Miyake. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 1981035-1042. - PMC - PubMed
-
- Bjerre, A., B. Brusletto, T. E. Mollnes, E. Fritzsonn, E. Rosenqvist, E. Wedege, E. Namork, P. Kierulf, and P. Brandtzaeg. 2002. Complement activation induced by purified Neisseria meningitidis lipopolysaccharide (LPS), outer membrane vesicles, whole bacteria, and an LPS-free mutant. J. Infect. Dis. 185220-228. - PubMed
-
- Brandtzaeg, P. 2006. Pathogenesis and pathophysiology of meningococcal disease, p. 427-480. In M. Frosch and M. Maiden (ed.), Handbook of meningococcal disease. Wiley-VCH Verlag, Weinheim, Germany.
-
- Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159195-204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

