A Candida albicans mannoprotein deprived of its mannan moiety is efficiently taken up and processed by human dendritic cells and induces T-cell activation without stimulating proinflammatory cytokine production
- PMID: 18591233
- PMCID: PMC2519437
- DOI: 10.1128/IAI.00669-08
A Candida albicans mannoprotein deprived of its mannan moiety is efficiently taken up and processed by human dendritic cells and induces T-cell activation without stimulating proinflammatory cytokine production
Abstract
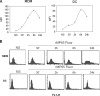
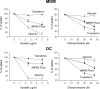
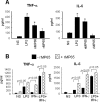
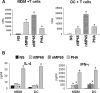
Mannoproteins are cell wall components of pathogenic fungi and play major virulence and immunogenic roles with both their mannan and protein moieties. The 65-kDa mannoprotein (MP65) of Candida albicans is a beta-glucanase adhesin recognized as a major target of the human immune response against this fungus, and its recombinant product (rMP65; devoid of the mannan moiety) is presently under consideration as a vaccine candidate. Here we investigated cellular and molecular aspects of the interaction of rMP65 with human antigen-presenting cells. We also assessed the ability of rMP65 to initiate a T-cell response. Both the native mannosylated MP65 (nMP65) and the recombinant product were efficiently bound and taken up by macrophages and dendritic cells. However, contrarily to nMP65, rMP65 did not induce tumor necrosis factor alpha and interleukin-6 release from these cells. On the other hand, rMP65 was rapidly endocytosed by both macrophages and dendritic cells, in a process involving both clathrin-dependent and clathrin-independent mechanisms. Moreover, the RGD sequence inhibited rMP65 uptake to some extent. After internalization, rMP65 partially colocalized with lysosomal membrane-associated glycoproteins 1 and 2. This possibly resulted in efficient protein degradation and presentation to CD4(+) T cells, which proliferated and produced gamma interferon. Collectively, these results demonstrate that the absence of the mannan moiety does not deprive MP65 of the capacity to initiate the pattern of cellular and molecular events leading to antigen presentation and T-cell activation, which are essential features for further consideration of MP65 as a potential vaccine candidate.
Figures



References
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. - PubMed
-
- Calderone, R., S. Suzuki, R. Cannon, T. Cho, D. Boyd, J. Calera, H. Chibana, D. Herman, A. Holmes, H. W. Jeng, H. Kaminishi, T. Matsumoto, T. Mikami, J. M. O'Sullivan, M. Sudoh, M. Suzuki, Y. Nakashima, T. Tanaka, G. R. Tompkins, and T. Watanabe. 2000. Candida albicans: adherence, signaling and virulence. Med. Mycol. 38(Suppl. 1)125-137. - PubMed
-
- Cassone, A. 2008. Fungal vaccines: real progress from real challenges. Lancet Infect. Dis. 8114-124. - PubMed
-
- Ciervo, A., F. Mancini, and A. Cassone. 2007. Transcription, expression, localization and immunoreactivity of Chlamydophila pneumoniae phospholipase D protein. Microb. Pathol. 4396-105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

