Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor h-binding protein
- PMID: 18591239
- PMCID: PMC2519416
- DOI: 10.1128/IAI.00367-08
Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor h-binding protein
Abstract
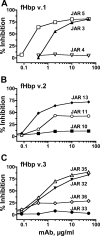
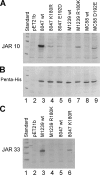
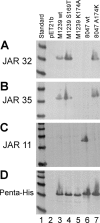
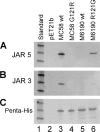
No broadly protective vaccine is available for the prevention of group B meningococcal disease. One promising candidate is factor H-binding protein (fHbp), which is present in all strains but often sparsely expressed. We prepared seven murine immunoglobulin G monoclonal antibodies (MAbs) against fHbp from antigenic variant group 2 (v.2) or v.3 ( approximately 40% of group B strains). Although none of the MAbs individually elicited bactericidal activity with human complement, all had activity in different combinations. We used MAb reactivity with strains expressing fHbp polymorphisms and site-specific mutagenesis to identify residues that are important for epitopes recognized by six of the v.2 or v.3 MAbs and by two v.1 MAbs that were previously characterized. Residues affecting v.2 or v.3 epitopes resided between amino acids 174 and 216, which formed an eight-stranded beta-barrel in the C domain, while residues affecting the v.1 epitopes included amino acids 121 and 122 of the B domain. Pairs of MAbs were bactericidal when their respective epitopes involved residues separated by 16 to 20 A and when at least one of the MAbs inhibited the binding of fH, a downregulatory complement protein. In contrast, there was no cooperative bactericidal activity when the distance between residues was >or=27 A or <or=14 A, which correlated with the inhibition of the binding of one MAb by the other MAb. Thus, a model for anti-fH MAb bactericidal activity against strains expressing low levels of fHbp requires the binding of two MAbs directed at nonoverlapping epitopes, which activates the classical complement pathway as well as inhibits fH binding. The latter increases the susceptibility of the organism to complement-mediated bacteriolysis.
Figures


References
-
- Beernink, P. T., J. A. Welsch, L. H. Harrison, A. Leipus, S. L. Kaplan, and D. M. Granoff. 2007. Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine. J. Infect. Dis. 1951472-1479. - PMC - PubMed
-
- Cantini, F., S. Savino, M. Scarselli, V. Masignani, M. Pizza, G. Romagnoli, E. Swennen, D. Veggi, L. Banci, and R. Rappuoli. 2006. Solution structure of the immunodominant domain of protective antigen GNA1870 of Neisseria meningitidis. J. Biol. Chem. 2817220-7227. - PubMed
-
- Capecchi, B., D. Serruto, J. Adu-Bobie, R. Rappuoli, and M. Pizza. 2004. The genome revolution in vaccine research. Curr. Issues Mol. Biol. 617-27. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

